Effect of TeO2 addition on the gamma radiation shielding competence and mechanical properties of boro-tellurite glass- an experimental approach
advertisement

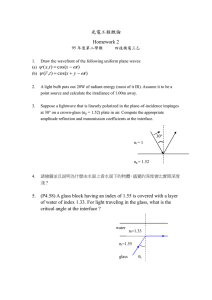
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/359020595 Effect of TeO2 addition on the gamma radiation shielding competence and mechanical properties of boro-tellurite glass: an experimental approach Article in Journal of Materials Research and Technology · March 2022 DOI: 10.1016/j.jmrt.2022.02.130 CITATIONS READS 11 1,527 12 authors, including: Mohammad Hasan Abu Mhareb Ashwitha Nancy D'Souza Imam Abdulrahman Bin Faisal University Manipal Academy of Higher Education 97 PUBLICATIONS 1,870 CITATIONS 10 PUBLICATIONS 70 CITATIONS SEE PROFILE Nouf Almousa Massachusetts Institute of Technology 34 PUBLICATIONS 173 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: enhancement of solar cell efficiency View project Glasses for radiation shielding View project All content following this page was uploaded by Fahad Ibraheem Almasoud on 20 March 2022. The user has requested enhancement of the downloaded file. SEE PROFILE j o u r n a l o f m a t e r i a l s r e s e a r c h a n d t e c h n o l o g y 2 0 2 2 ; 1 8 : 1 0 1 7 e1 0 2 7 Available online at www.sciencedirect.com journal homepage: www.elsevier.com/locate/jmrt Original Article Effect of TeO2 addition on the gamma radiation shielding competence and mechanical properties of boro-tellurite glass: an experimental approach M.I. Sayyed a,b,*, Nidal Dwaikat c,d, M.H.A. Mhareb e,f, Ashwitha Nancy D'Souza g, Nouf Almousa h, Y.S.M. Alajerami i, Fahad Almasoud j,k, K.A. Naseer l,**, Sudha D. Kamath g, Mayeen Uddin Khandaker m, Hamid Osman n, Sultan Alamri n a Department of Physics, Faculty of Science, Isra University, Amman, Jordan Department of Nuclear Medicine Research, Institute for Research and Medical Consultations (IRMC), Imam Abdulrahman Bin Faisal University (IAU), Dammam, Saudi Arabia c Department of Physics, College of Engineering and Physics, King Fahd University of Petroleum & Minerals, Dhahran, 31261, Saudi Arabia d Interdisciplinary Research Center for Advanced Materials, King Fahd University of Peroleum & Minerals, Dhahran, Saudi Arabia e Department of Physics, College of Science, Imam Abdulrahman Bin Faisal University, P.O. Box 1982, 31441, Dammam, Saudi Arabia f Basic and Applied Scientific Research Center, Imam Abdulrahman Bin Faisal University, PO Box 1982, 31441, Dammam, Saudi Arabia g Department of Physics, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, India h Department of Physics, College of Science, Princess Nourah Bint Abdulrahman University, P.O. Box 84428, Riyadh, 11671, Saudi Arabia i Medical Imaging Department, Applied Medical Sciences Faculty, Al Azhar University-Gaza, Palestine j Nuclear Science Research Institute (NSRI), King Abdulaziz City for Science and Technology (KACST), Riyadh, 11442, Saudi Arabia k Department of Soil Sciences, College of Food and Agricultural Sciences, King Saud University, Riyadh, 12372, Saudi Arabia l Department of Physics, Farook College (Autonomous), Kozhikode, 673632, India m Centre for Applied Physics and Radiation Technologies, School of Engineering and Technology, Sunway University, 47500, Bandar Sunway, Selangor, Malaysia n Department of Radiological Sciences, College of Applied Medical Sciences, Taif University, Taif 21944, Saudi Arabia b article info abstract Article history: We experimentally investigated the effect of TeO2 on the radiation-shielding competence Received 3 January 2022 of a BaOeMoO3eB2O3 glass system. Two gamma-ray sources (137Cs and Accepted 27 February 2022 scintillator detector (sodium iodide (NaI(Tl)) were utilized to measure the attenuation Available online 4 March 2022 factors of the prepared glass at 0.184, 0.280, 0.662, 0.710, and 0.810 MeV. The measured 166 Ho) and a * Corresponding author. ** Corresponding author. E-mail addresses: dr.mabualssayed@gmail.com (M.I. Sayyed), naseerka.phy6@gmail.com (K.A. Naseer). https://doi.org/10.1016/j.jmrt.2022.02.130 2238-7854/© 2022 The Authors. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/). 1018 j o u r n a l o f m a t e r i a l s r e s e a r c h a n d t e c h n o l o g y 2 0 2 2 ; 1 8 : 1 0 1 7 e1 0 2 7 Keywords: mass attenuation coefficient agreed well with the theoretically calculated values for all the Boro-tellurite glass prepared samples. The linear attenuation coefficient (LAC) results demonstrated that as Radiation shielding the photon energy increased, the penetrating ability of the photons through the glass Scintillator detector increased. The LAC values of boro-tellurite glass at 662 keV were compared with those of Tenth-value layer other tellurite glass. We found that MTB1 glass produced better attenuation results than 10Li2Oe20K2Oe50B2O3e20TeO2 glass, whereas MTB5 glass with 70 mol% TeO2 had an LAC value greater than that of 90.4TeO2-9.6ZnOe4NiO glass. The half-value layer (HVL) increased continuously with photon energy. For MTB1 glass, the HVL increased from 0.3609 cm at 184 keV to 1.6078 cm at 662 keV and 1.8381 cm at 810 keV. The lowest set of HVL values was observed for MTB5 glass, which confirmed its superior attenuation properties compared to other compositions. The transmission factor (TF) was also calculated; MTB5 glass had the lowest TF values, which revealed that MTB5 provided the best shield. For glass with a thickness of 1 cm, the TF was 75.8% for MTB1, 72.8% for MTB2, 70.6% for MTB3, 68.8% for MTB4, and 63.4% for MTB5. © 2022 The Authors. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). 1. Introduction Human radiation exposure can occur in nuclear research facilities, nuclear reactors, during radiological diagnoses, and in various other applications. High-energy ionizing radiation can be extremely harmful to human tissue and living cells because it ionizes the atoms it comes in contact with by ripping away their electrons. To safely harness the benefits of radiation, radiation shields are placed between the radiation source and the person or object that needs to be protected [1e5]. The creation of shields that absorb gamma radiation has attracted the interest of researchers in the field because of the high penetration ability of this form of radiation. The field of radiation shielding strives to prevent nuclear accidents and radiation leaks in the future and ensure safe transportation and storage of nuclear waste. An effective radiation shield should be defined by its ability to protect against these forms of harmful radiation [6e10]. A variety radiation shields are used, depending on the requirements of the desired implementation. Concrete is a common shielding substance owing to its low cost and versatility. Although concrete offers multiple advantages as a radiation shield, it tends to develop cracks over long periods of exposure to radiation because of the tensile stress produced, loses water content due to heating, and is unable to allow visible light transmission. Owing to these general disadvantages, researchers have attempted to discover alternative materials that can more effectively shield against incoming photons [11,12]. Recently, glass has gained immense attention because of its transparency to visible light, which is a property that other shielding materials lack. Additionally, the composition of the glass system can be varied significantly by doping different heavy metal oxides (HMOs) into the glass matrix [13e16]. By introducing these HMOs into glass, their radiation shielding properties are greatly enhanced, and the glass can absorb gamma rays and neutrons more effectively. There are three types of HMOs that act differently on the glass matrix: glass network formers, glass network modifiers, and glass intermediates. When glass formers are introduced into a glass system, they form the backbone of the network, whereas glass modifiers alter the glass network by creating non-bridging oxygen but are not part of the network's backbone. Glass intermediates have properties similar to those of formers and modifiers, and these properties vary depending on the composition of the glass matrix [17e20]. Tellurite is a glass intermediate with great promise in the radiation-shielding field owing to its high thermal stability, high density, high electrical conductivity, low melting temperature, and significant moisture and corrosion resistance [21,22]. By itself, TeO2 cannot form a glass system and requires additional glass modifiers to form stable glass. Other HMOs such as PbO, MoO3, WO3, BaO, and Bi2O3 can be added to tellurite glass to improve their optical, thermal, and mechanical properties [23e27]. For instance, MoO3, can be a helpful additive because it can act as either a glass modifier or glass former. HMOs are beneficial in radiation shielding because they tend to increase the density of the glass system, have a broad attenuation cross-section, and maintain good optical and mechanical properties [28,29]. TeO2 is a heavy glass former, and it has been used in radiation shielding because of its high ability to absorb gamma rays compared with other glass formers. Tijani et al. [30] studied the shielding features of transparent tellurite glass within diagnostic energy ranges of 20, 30, 40, and 60 keV. The radiation-shielding features were evaluated using the XCOM program, and the results obtained for the glass samples were compared with those of the concrete samples. The fabricated glass exhibited better shielding results than those of the concrete samples. Al-Buriahi et al. [31] reported the radiation shielding and mechanical features of (100-x)TeO2-xZnO-4NiO glass. The radiation shielding properties were investigated theoretically using the Phy-X program and Geant4 simulation, and the studied parameters included the mass attenuation coefficient (m/r) and transmission factor (TF). The addition of zinc oxide (ZnO) to the glass system directly affected the mechanical and radiation shielding properties. Rammah et al. [32] evaluated the radiation-shielding capacity of a new group of tellurite glass systems. The glass system contained both vanadium and antimonates. The impact of antimony oxide j o u r n a l o f m a t e r i a l s r e s e a r c h a n d t e c h n o l o g y 2 0 2 2 ; 1 8 : 1 0 1 7 e1 0 2 7 Table 1 e The composition ratio and density values of 20BaOeMoO3-(70-x)B2O3:xTeO2. Sample codes MTB1 MTB2 MTB3 MTB4 MTB5 Composition ratio (mole%) BaO MoO3 B2O3 TeO2 20 20 20 20 20 10 10 10 10 10 70 52.5 35 17.5 0 0 17.5 35 52.5 70 Density (g cm3) 3.4446 3.9320 4.3091 4.6314 5.5057 1019 explored for fabricated glass. The addition of Sb2O3 to the tellurite glass system led to an increase in the linear attenuation coefficient (LAC), whereas the fast neutron removal crosssection (SR) reduced with increasing Sb2O3 content. Sayyed et al. [27] reported the radiation shielding of a novel tellurite glass system ((60-x)TeO2-10GeO2-20ZnOe10BaO-xBi2O3). MCNP-5 was used to study radiation-shielding features within energies ranging from 0.015 to 15 MeV. The replacement of TeO2 with bismuth oxide (Bi2O3) enhanced the gammashielding features and reduced SR. Numerous studies have been conducted on tellurite glass in the radiation-shielding field [9,21,22,24,26,27,33]. In this study, we experimentally investigated the influence of TeO2 on the radiation-shielding competence of a BaOeMoO3eB2O3eTeO2 glass system. 2. Materials and methods 2.1. Glass fabrication A series of boro-tellurite glasses were prepared using the traditional melt-quenching process, and different raw oxides such as tellurium oxide (TeO2), boron oxide (B2O3), barium oxide (BaO), and molybdenum oxide (MoO3) were utilized for this purpose. These oxides were determined at specific ratios, as listed in Table 1. The raw oxides were weighed carefully, mixed to obtain a homogenous mixture, and placed in an alumina crucible. Subsequently, the homogenous mixture was introduced into an electrical furnace at 1100 C for 40 min and stirred periodically to discharge bubbles during the melting process. The molten mixture was then annealed inside another electrical furnace at 450 C for 3 h, and a steel plate was used to pour the molten mixture. The same procedure has been applied to other types of glass systems [33,34]. 2.2. Fig. 1 e Photograph of the experimental setup. (Sb2O3) on the radiation shielding features was studied using WinXcom and a Monte Carlo simulation code (MCNP- 5) within an energy range of 15 keV to 15 MeV. The projected range and stopping power for protons and alpha particles were also Glass density The Archimedes concept was employed to determine the current glass, and purified water was used as the submersion liquid. A Radwag balance with a particular density kit system was utilized for this purpose. The samples were weighed in air (A) and fluid (B) to calculate the density (r) experimentally, and the following relation was employed: r¼ A : AB ð1Þ Fig. 2 e Schematic diagram of the detection system. 1020 ( ( ( ( ( j o u r n a l o f m a t e r i a l s r e s e a r c h a n d t e c h n o l o g y 2 0 2 2 ; 1 8 : 1 0 1 7 e1 0 2 7 Fig. 3 e The experimental and XCOM mass attenuation coefficients of the studied glass. 2.3. Experimental radiation shielding A well-designed irradiation setup at the King Fahd University of Petroleum & Minerals (KFUPM) was used to investigate the gamma-radiation shielding properties of the materials. It consists of a narrow gamma-ray beam collimator (three collimation steps) and gamma-ray spectrometer, as shown in Fig. 1. The spectrometer is composed of a (300 300 ) NaI (Tl) detector, preamplifier, high-voltage power supply, amplifier (Ortec 572 A), 8 k multichannel analyzer (Amptek MCA8000D), and computer (see Fig. 2). The detector is housed with layers of 10-cm thick lead bricks to minimize the background. The resolution (FWHM) of the detector at 662 keV of 137Cs is 7%. The collimator was fabricated by drilling three apertures of different sizes in the center of the three lead bricks (see Fig. 1). The centers of the apertures were aligned with the center of the detector using a laser pointer. As described in a previous publication [35], the chi-squared test was used to check the performance of the detection system. In this study, two familiar radioactive sources, Cs-137 (E_g ¼ 662 keV) with an activity of 16.6 kBq and Ho-166 m (E_g ¼ 184, 280, 710, and 810 keV) with an activity of 29.6 kBq were used to calibrate the system and investigate the gamma shielding property of glass samples. The gamma spectra of the Cs-137 and Ho-166 m sources were recorded with and without the samples for 1 h using the previously mentioned MCA, and DPPMCA computer-based software was used to analyze the spectra. The background was measured during the same period. After the subtraction of the background, the area under the photopeak (gross count) of the gamma line transitions at 662 (Cs-137), 184, 280, 710, 0.55 0.50 2.4 MTB1 MTB2 MTB3 MTB4 MTB5 1.2 0.8 0.35 0.30 0.25 0.4 0.0 100 0.40 -1 1.6 0.45 LAC (cm ) LAC (cm-1) 2.0 0.20 200 300 400 500 600 700 800 900 Photon energy (keV) Fig. 4 e The linear attenuation coefficient of the studied glass. -10 0 10 20 30 40 50 60 70 80 TeO2 (mol%) Fig. 5 e Comparison of the linear attenuation coefficient of the studied glass and other tellurite glass in literature. 1021 j o u r n a l o f m a t e r i a l s r e s e a r c h a n d t e c h n o l o g y 2 0 2 2 ; 1 8 : 1 0 1 7 e1 0 2 7 and 810 keV (Ho-166 m), with a 95% confidence level, was measured and used to calculate the linear attenuation coefficient (m) using 2.4 LAC ; MAC ¼ r ln 2 ; LAC ð4Þ MFP ¼ 1 ; LAC ð5Þ TVL ¼ ln10 ; LAC ð6Þ RBE ¼ 1 eLAC x t x 100ð%Þ; ð7Þ ð8Þ where t is the thickness of the glass. The mass stopping power (MSP) and projected range (PR) were evaluated using the SIRM program [36], and the removal cross-section of fast neutrons (SR) was determined using the Phy-X program [37]. 3. Results and discussion 3.1. Radiation shielding features Table 1 shows the chemical composition, corresponding sample codes, and density values of the synthesized borotellurite glass. The density values were found to increase with the successive addition of TeO2, which is a consequence of replacing B2O3 molecules of lower molecular weight (M ¼ 69.63 g/mol) with TeO2 molecules (M ¼ 159.6 g/mol). Figure 3 shows a comparative representation of m/r determined experimentally and theoretically from the XCOM database. It is clear that the values measured from both methods agreed well for all glass samples at all energies, with minimal difference between the values. With the help of theoretical m/r values, another essential parameter, the LAC, was calculated by multiplying with the density values. The computed LAC values (Fig. 4) also showed a trend similar to that of m/r. As the photon energy increased, the LAC values decreased for glass of all compositions. This is MTB1 MTB2 MTB3 MTB4 MTB5 2.0 1.6 1.2 0.8 0.4 0.0 200 400 600 800 Photon energy (keV) ð3Þ HVL ¼ I x100 ¼ eLAC x t x 100ð%Þ; Io HVL (cm) ð2Þ where x is the thickness of the sample (cm), which ranges from 0.199 to 0.496 cm for MTB1 to MTB5, m is the linear attenuation (cm1), I0 is the photopeak area at specified energy lines after the subtraction of the background with no sample between the source and the detector, and I is the photopeak area after subtraction of the background with the sample between the source and the detector. Based on the LAC results, the tenth value layer (TVL), mean free path (MFP), half-value layer (HVL), radiation protection efficiency (RBE), mass attenuation coefficient (MAC), and TF were obtained. TF ¼ 2.8 Fig. 6 e The half value layer of the studied glass. caused by the penetration of high-energy photons into the material with greater ease and without interacting with matter, which in turn decreases the probability of photon interaction. However, the trend followed by the decreasing LAC values can be split into two parts, one at low and the other at high energies. The sudden decrease observed at lower energies is due to the photoelectric absorption of photons by the glass material. Furthermore, at higher energies, the LAC decreased at a slower rate because of the dominance of the Compton scattering process [38e40]. Figure 4 also shows that at each energy, the LAC increased with an increase in TeO2 concentration, and MTB5 glass showed the highest attenuation property. This was purely due to the contribution of TeO2 in enhancing the molecular weight and density of the glass samples. Figure 4 clearly shows the increasing LAC values at different photon energies with increasing number of TeO2 molecules. In Fig. 5, the LAC values of boro-tellurite glass at 662 keV are compared with those of other tellurite glass found in literature [25,31,38,41e44]. All the prepared glass have higher LACs and thus better shielding ability than 10Li2Oe20K2Oe50B2O3e20TeO2 [41] and 39.5B2O3e25TeO2e15BaOe10Na2 10 9 8 TVL (cm) I ¼ Io exm ; 3.2 MTB1 MTB2 MTB3 MTB4 MTB5 7 6 5 4 3 2 1 0 200 400 600 800 Photon energy (keV) Fig. 7 e The tenth value layer of the studied glass. 1022 j o u r n a l o f m a t e r i a l s r e s e a r c h a n d t e c h n o l o g y 2 0 2 2 ; 1 8 : 1 0 1 7 e1 0 2 7 4.8 184 280 662 720 810 4.2 MFP (cm) 3.6 3.0 2.4 1.8 1.2 0.6 0.0 3.0 3.5 4.0 4.5 5.0 5.5 3 Density (g/cm ) Fig. 8 e The mean free path of the studied glass as a function of density. Oe10K2O-0.5Dy2O3 [42] glass. MTB5 (glass with 70 mol% TeO2) has an LAC close to that of 10WO3e10MoO3e80TeO2 [38] glass and a slightly higher LAC than the 90.4TeO2-9.6ZnOe4NiO [31] sample. Additionally, MTB5 glass has a higher LAC value than 10SrOe25B2O3e30TeO2e20BaOe15MoO3 [25] and 60TeO2e10SrOe30B2O3 [44] glass, but a lower LAC than the 29.5B2O3e30TeO2e20PbF2e20PbO-0.5Dy2O3 [43] sample. This comparison demonstrates the importance of developing glass with relatively high amounts of TeO2 to obtain glass with better radiation attenuation properties than the available boro-tellurite glass. The HVL and TVL are considered essential shielding parameters for evaluating fabricated glass. The influence of photon energy on the HVL and TVL values was analyzed with the help of the graphs shown in Figs. 6 and 7. It is known that glass with lower HVL and TVL values can block radiation photons with minimal thickness. In Figs. 6 and 7, both parameters increase continuously with photon energy. For MTB1 glass, the HVL increased from 0.3609 cm at 184 keV to 1.6078 cm at 662 keV and 1.8381 cm at 810 keV. This indicates Fig. 9 e The transmission factor of the prepared glass. Fig. 10 e The radiation protection efficiency of the prepared glass. that a greater thickness is required for the attenuation of highenergy photons. In addition, in terms of TeO2 content, the HVL decreased from 2.772 cm to 2.5018 cme1.8381 cm for MTB1, MTB3, and MTB5, respectively. Hence, the lowest set of HVL values observed for MTB5 glass confirmed its superior attenuation properties compared to other compositions. Figure 8 shows a graph of the MFP values as a function of density at different photon energies. As the density increased, the MFP values decreased in each case, indicating the significance of adding TeO2 molecules to the glass. The minimum MFP values exhibited at an energy of 0.184 MeV suggest that the capacity of the studied glass to attenuate low-energy photons is greater than that of high-energy photons. Because the density value of 5.5057 g cm3 produced the lowest MFP values at all Fig. 11 e The projected range of the prepared glass. j o u r n a l o f m a t e r i a l s r e s e a r c h a n d t e c h n o l o g y 2 0 2 2 ; 1 8 : 1 0 1 7 e1 0 2 7 Fig. 12 e The mass stopping power of the prepared glass. energies, the corresponding MTB5 glass can act as a better shielding agent than the others. The number of photons that can penetrate MTB glass can be estimated using the TF. The TF shows the ratio of the real number of photons transmitted from the first side of the glass to the number of photons emitted from the other side of the radioisotope source. Accordingly, to develop novel shields, we attempted to identify a sample with a low TF. For the current MTB glass, we present the results of the TF for five thicknesses (1e5 cm) in Fig. 9, which clearly shows the role of the sample composition and its thickness on the TF. First, MTB5 was the best attenuator, according to Fig. 9, because it has the lowest TF values. In contrast, MTB1 was the worst shield owing to its high TF. These results are consistent with those concluded 1023 from previous figures. Owing to the high quantity of TeO2 (70 mol%), the chances of collision between photons and the atoms of MTB5 are high. Therefore, most photons are absorbed or scattered by MTB5, and the number of photons transmitted from the first side to the other side is small; hence, the TF value is low. Regarding the effect of thickness on the TF for a certain composition, it is evident that increasing the thickness has an inverse influence on the TF values. For a glass with a thickness of 1 cm, the TF was 75.8% for MTB1, 72.8% for MTB2, 70.6% for MTB3, 68.8% for MTB4, and 63.4% for MTB5, and these values decreased to 57.5%, 53.0%, 49.8%, 47.3%, and 40.2%, respectively, for the aforementioned glass with a thickness of 2 cm. The TF results indicate that, to develop glass with the oxides used in this study and with the same concentrations given in Table 1, we must select a thickness greater than 3 cm. In this case, the TF was less than 30% (except for MTB1, where the TF for a thickness of 4 cm was 33%). Therefore, glass with a high quantity of TeO2 (more than 50 mol%) and containing 20 mol% of BaO and 10 mol% of MoO3 can attenuate most of the incoming radiation if the thickness exceeds 3 cm. Figure 10 shows the radiation protection efficiency (RPE) of the MTB glass at different thicknesses; this can be used to confirm the relationship between the thickness of the glass and their attenuation ability. The RPE increased rapidly as the thickness of the glass increased from 1 cm to 5 cm; this is a result of the increase in photon interaction with the atoms as the thickness increases, which boosts the attenuation performance of the sample. For MTB1, the RPE was approximately 24% at 1 cm, which increased to approximately 75% at a thickness of 5 cm. In addition to thickness, the composition of the glass also affected the RPE. MTB5 glass was the best attenuator owing to its high RPE. At 4 and 5 cm, the RPE was greater than 75%, suggesting that most photons cannot pass through high-thickness MTB5. Figures 11 and 12 display the MSP and PR of protons, respectively. From Fig. 11, the distance range of the proton particles inside the glass samples can be determined before their energy is deposited. The PR of protons was higher for the Fig. 13 e The neutron fast removal section (SR) of (a) the fabricated glass and (b) the MTB4 and MTB5 samples in comparison with different materials such as PLPG3, ordinary concrete (OC), and PLPG0. 1024 j o u r n a l o f m a t e r i a l s r e s e a r c h a n d t e c h n o l o g y 2 0 2 2 ; 1 8 : 1 0 1 7 e1 0 2 7 Table 2 e Elastic properties of the studied glass. Elastic properties Young's modulus (E in GPa) Bulk modulus (K in GPa) Shear modulus (G in GPa) Longitudinal modulus (L in GPa) Hardness (H in GPa) Fractal bond connectivity (d) Total packing density (Vt) Poisson's ratio (s) MTB1 MTB2 MTB3 MTB4 MTB5 56.20 76.84 101.36 129.68 157.88 24.07 47.03 87.24 157.81 280.13 25.30 31.29 38.80 47.57 56.14 57.80 88.76 138.97 221.23 354.99 6.559 5.673 5.019 4.345 3.519 4.205 2.661 1.779 1.206 0.802 0.357 0.510 0.717 1.014 1.479 0.111 0.228 0.306 0.363 0.406 MTB1 samples than for the other samples, and the PR decreased with decreasing TeO2 content. For example, the PR values at 0.01 MeV were 2.0828, 1.9285, 1.7692, 1.6535, and 1.5032 mm for MTB1, MTB2, MTB3, MTB4, and MTB5, respectively. Additionally, the effect of kinetic energy on the PR values is clear; the PR values at 10 and 1 MeV for MTB5 were 1015 and 58.5443 mm, respectively. The increase in kinetic energy for the proton particles led to a higher penetration of protons inside the glass samples. Figure 12 illustrates the relationship between the kinetic energy and MSP for the proton particles of the fabricated glass. At 0.8 MeV, the MSP values were higher, and the MSP for the MTB1 sample was superior to that of the other samples owing to the inverse dependence of MSP on density. The MTB1 sample had the lowest density compared to the other samples, and the compatibility between the density and MSP led to these results. The MSP values for the samples under study at 10 MeV were 1.5499, 1.4351, 1.3166, 1.2191, and 0.8127 MeV cm2/g, whereas the MSP for lead (Pb) and water at the same energy was 17.5 and 47 MeV cm2/g. In general, heavy atoms and compounds have a lower ability to slow down charged particles (protons) because they tie their electrons strongly with the inner shell, reducing the ability to absorb charged particles. Thus, it can be concluded that MTB5 has the highest efficiency for stopping protons. The neutron fast removal section (SR) results for the fabricated glass are shown in Fig. 13-a. Fig. 13-b shows that the MTB4 and MTB5 samples were compared with various substances such as PLPG3, ordinary concrete (OC), and PLPG0. Fig. 13-a shows that the values of SR decrease with increasing TeO2 to glass system up to MTB4; after this, an enhancement of the SR values can be observed for MTB5. The glass systems for MTB1 and MTB5 are different because MTB1 is based on borate, which is suitable for absorbing neutrons, whereas the MTB5 sample is based on tellurate, which is less capable of absorbing neutrons than borate. The mixing between borate and tellurate in this glass system led to a reduction in SR, as evident in samples MTB2, MTB3, and MTB4. This reduction was expected owing to the replacement of the high-ability neutron absorber (B) with a lower-ability neutron absorber (Te). In contrast, Fig. 13-b displays SR for MTB4, MTB5, and different materials. The results show the superiority of MTB4 and MTB5 over other materials, indicating the excellent ability of current glass to absorb neutrons. 3.2. Elastic properties The investigation of the elastic properties of glass emerges from the dependence of these properties on the organization and underlying conservation of the glass network. These also 350 150 250 100 200 150 100 50 50 Longitudinal modulus (L in GPa) & Bulk modulus (K in GPa) Young's modulus (E in GPa) & Shear modulus (G in GPa) 300 0 0.0 17.5 35.0 52.5 70.0 TeO2 content (mol %) Fig. 14 e The variation in the Young's modulus (E), shear modulus (G), bulk modulus (K) and longitudinal modulus (L) of the studied glass. 1025 6.5 0.40 6.0 0.35 5.5 0.30 5.0 0.25 4.5 0.20 4.0 0.15 3.5 Poisson's ratio ( ) Hardness (H in GPa) j o u r n a l o f m a t e r i a l s r e s e a r c h a n d t e c h n o l o g y 2 0 2 2 ; 1 8 : 1 0 1 7 e1 0 2 7 0.10 0.0 17.5 35.0 52.5 70.0 TeO2 content (mol %) Fig. 15 e The hardness and Poisson's ratio with respect to TeO2 content. determine the mechanical conduct of the glass and provide data regarding the compound's glass construction and solidstate movement related to the interatomic bonds inside the setup [45,46]. In particular, they react to geometrical and arrangement changes due to the doping of modifiers [47,48]. The versatile properties, which are broadly consistent, show significant changes over the glass transition temperature because, above this transition temperature, the network disintegrates, and the non-elastic nature decreases. Examining the elastic moduli of the glass structure plays a significant role in dissecting the glass transition [49]. Utilizing theoretical models gathered from literature [50e52], elastic constants (E, K, G, L), the total packing density (Vt), hardness (H), molar atomic volume (Va), fractal bond connectivity, and Poisson's ratio (s) were estimated for the fabricated glass to obtain a detailed picture of their design, as shown in Table 2. These parameters were assessed as elements of the glass arrangement, packing density, and dissociation energy per unit volume (Gi) of the glass. Typical values of dissociation energy were obtained from literature [47]. The calculated s values noted in the range 0.111e0.406 exhibited an increase from the low-to high-order glass as a result of the expanded compaction concentration. The 20NBDB glass had a lower s value, which indicates a measurable effect on s. Therefore, from s, the hardness (H) of the glass was assessed, which decreased with an increase in the amount of TeO2, as shown in Fig. 14. The elastic constants, represented by the intermediate-range structural units (fragility) of the glass, were estimated. The extra oxygen added through the modifiers delivers superstructural units of borate, which leads to the development of NBOs in the glass design. As the amount of TeO2 increased, the moduli E, K, G, and L increased, indicating the production of BOs in the glass framework. The overall increase in the elastic constants of the concentrated glass is shown in Fig. 15. Fractal bond connectivity (d) is a significant device for examining the adjustment of the dimensionality of glass. 4. Conclusion We reported the influence of TeO2 on MAC, Zeff, and other shielding factors of a BaOeMoO3eB2O3 glass system using a scintillator detector and two gamma-ray sources (137Cs and 166 Ho). In addition, we report the influence of glass thickness on the TF. The LAC values decreased as the energy increased from 0.184 to 0.81 MeV for all glass compositions; hence, the radiation blocking competence of glass was weakened by increasing the energy. At 0.662 MeV, the LAC value of the MTB5 glass was more significant than that of the 90.4TeO2-9.6ZnOe4NiO glass and slightly lower than that of the 10WO3e10MoO3e80TeO2 glass. The minimum MFP values were found for an energy of 0.184 MeV, which suggests that these glass are capable of absorbing low-energy photons better than high-energy photons. The TF values indicate that MTB5 was the best attenuator, whereas MTB1 was the worst shield. The main conclusion of this study is that glass with a high quantity of TeO2 (more than 50 mol%) and containing 20 mol% of BaO and 10 mol% of MoO3 can attenuate most of the incoming radiation if the thickness exceeds 3 cm. Extremely dense MTB glass were found to possess elevated E, K, L, and G values, indicating their better cross-linked structure. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. 1026 j o u r n a l o f m a t e r i a l s r e s e a r c h a n d t e c h n o l o g y 2 0 2 2 ; 1 8 : 1 0 1 7 e1 0 2 7 Acknowledgement [14] We deeply acknowledge Taif University for supporting the researchers through Taif University Researchers Supporting Project number (TURSP-2020/287), Taif University, Taif, Saudi Arabia. The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R111), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. [15] [16] references [17] [1] Dong M, Zhou S, Xue X, Feng X, Sayyed MI, Khandaker MU, et al. The potential use of boron containing resources for protection against nuclear radiation. Radiat Phys Chem 2021;188:109601. https://doi.org/10.1016/ j.radphyschem.2021.109601. [2] Dong M, Xue X, Yang H, Li Z. Highly cost-effective shielding composite made from vanadium slag and boron-rich slag and its properties, Radiat. Phys Chem 2017;141:239e44. https://doi.org/10.1016/j.radphyschem.2017.07.023. [3] Dong M, Xue X, Yang H, Liu D, Wang C, Li Z. A novel comprehensive utilization of vanadium slag: as gamma ray shielding material. J Hazard Mater 2016;318:751e7. https:// doi.org/10.1016/j.jhazmat.2016.06.012. rez S, Man ~ anes SA. Radiation [4] Tamayo P, Thomas C, Rico J, Pe shielding properties of siderurgical aggregate concrete. Construct Build Mater 2022;319:126098. [5] Sayyed MI, Rammah YS, Abouhaswa AS, Tekin HO, Elbashir BO. ZnO-B2O3-PbO glasses: synthesis and radiation shielding characterization. Phys B Condens Matter 2018;548:20e6. [6] Dong Mengge, Zhou Suying, Xue Xiangxin, Sayyed MI, Tishkevich Daria, Trukhanov Alex, et al. Study of comprehensive shielding behaviors of chambersite deposit for neutron and gamma ray. Prog. Nucl. Energy 2022;146:104155. https://doi.org/10.1016/j.ijleo.2021.166790. [7] Sayyed MI, Elmahroug Y, Elbashir BO, Issa Shams AM. Gamma-ray shielding properties of zinc oxide soda lime silica glasses. Jf Mater Sci: Electronic Mater 2017;28:4064e74. [8] Kurtulus R, Kurtulus C, Kavas T. Nuclear radiation shielding characteristics and physical, optical, mechanical, and thermal properties of lithium-borotellurite glass doped with Rb2O. Prog Nucl Energy 2021;141:103961. . lar I, Cengiz GB, Bilir G. Gamma radiation shielding [9] Çag properties of some binary tellurite glasses. J Non-Cryst Solids 2021;574:121139. [10] An JM, Lin H, Pun EYB, Li DS. Synthesis, gamma and neutron attenuation capacities of boron-tellurite glass system utilizing Phy-X/PSD database. Mater Chem Phys 2021;274:125166. € € Gamma-ray € kçe HS, Oztürk [11] Go BC, Çam NF, Andiç-Çakır O. attenuation coefficients and transmission thickness of high consistency heavyweight concrete containing mineral admixture. Cement Concr Compos 2018;92:56e69. https:// doi.org/10.1016/j.cemconcomp.2018.05.015. € kçe HS, Yalçınkaya Ç, Tuyan M. Optimization of reactive [12] Go powder concrete by means of barite aggregate for both neutrons and gamma rays. Construct Build Mater 2018;189:470e7. https://doi.org/10.1016/ j.conbuildmat.2018.09.022. [13] Issa Shams AM, Saddeek Yasser B, Sayyed MI, Tekin HO, Kilicoglu Ozge. Radiation shielding features using MCNPX code and mechanical properties of the PbO-Na2O-B2O3-CaO- [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] Al2O3-SiO2 glass systems. Composites Part B 2019;167:231e40. Singh S, Kaur R, Rani S, Sidhu BS. Investigations on physical, structural and nuclear radiation shielding behaviour of niobiumebismuthecadmiumezinc borate glass system. Prog Nucl Energy 2021;142:104038. Rajesh M, Kavaz E, D.P.R. B.. Photoluminescence, radiative shielding properties of Sm3þ ions doped fluoroborosilicate glasses for visible (reddish-orange) display and radiation shielding applications. Mater Res Bull 2021;142:111383. https://doi.org/10.1016/j.materresbull.2021.111383. Naseer KA, Marimuthu K. The impact of Er/Yb co-doping on the spectroscopic performance of bismuth borophosphate glasses for photonic applications. Vacuum 2021;183:109788. https://doi.org/10.1016/j.vacuum.2020.109788. Alajerami YS, Drabold D, Mhareb MHA, Cimatu KLA, Chen G, Kurudirek M. Radiation shielding properties of bismuth borate glasses doped with different concentrations of cadmium oxides. Ceram Int 2020;46:12718e26. https:// doi.org/10.1016/j.ceramint.2020.02.039. Kaur P, Singh D, Singh T. Heavy metal oxide glasses as gamma rays shielding material. Nucl Eng Des 2016;307:364e76. https:// doi.org/10.1016/j.nucengdes.2016.07.029. Al-Hadeethia Y, Sayyed MI. Analysis of borosilicate glasses doped with heavy metal oxides for gamma radiation shielding application using Geant4 simulation code. Ceram Int 2019;45:24858e64. Cheewasukhanont W, Limkitjaroenporn P, Kothan S, Kedkaew C, Kaewkhao J. The effect of particle size on radiation shielding properties for bismuth borosilicate glass. Radiat Phys Chem 2020;172:108791. https://doi.org/10.1016/ j.radphyschem.2020.108791. Sayyed MI, Mhareb MHA, Abbas ZY, Almousa N, Laariedh F, Kaky KM, et al. Structural, optical, and shielding investigations of TeO2eGeO2eZnOeLi2OeBi2O3 glass system for radiation protection applications. Appl Phys A 2019;125:417. https://doi.org/10.1007/s00339-019-2709-3. Almuqrin AH, Sayyed MI. Radiation shielding characterizations and investigation of TeO2eWO3eBi2O3 and TeO2eWO3ePbO glasses. Appl Phys A 2021;127:190. https://doi.org/10.1007/s00339-021-04344-9. Dwaikat Nidal, Sayyed MI, Mhareb MHA, Dong Mengge, Alajerami YSM, Alrammah Ibrahim, et al. Durability, optical and radiation shielding properties for new series of borotellurite glass. Optik 2021;245:167667. Kaur Arshpreet, Khanna Atul, Sathe Vasant G, Gonzalez Fernando, Ortiz Belen. "Optical, thermal, and structural properties of Nb2O5eTeO2 and WO3eTeO2 glasses. Phase Transitions 2013;86(6):598e619. Sayyed MI, El-bashir BO, Alhuthali Abdullah MS, Alajerami YSM, Al-Hadeethi Y, Mhareb MHA. "Gamma radiation shielding and structural features for barium strontium boro-tellurite glass modified with various concentrations of molybdenum oxide. J Non-Cryst Solids 2021;559:120658. Rao N Narasimha, Kityk IV, Kumar V Ravi, Raghava Rao P, Raghavaiah BV, Czaja P, et al. "Piezoelectric and elastic properties of ZnF2ePbOeTeO2: TiO2 glass ceramics. J NonCryst Solids 2012;358(3):702e10. Sayyed MI, Ati Ali A, Mhareb MHA, Mahmoud KA, Kaky Kawa M, Baki SO, et al. Novel tellurite glass (60-x) TeO2e10GeO220ZnOe10BaO-xBi2O3 for radiation shielding. J Alloys Compd 2020;844:155668. Chanthima N, Kaewkhao J, Limsuwan P. Study of photon interactions and shielding properties of silicate glasses containing Bi2O3, BaO and PbO in the energy region of 1keV to 100GeV. Ann Nucl Energy 2012;41:119e24. https://doi.org/ 10.1016/j.anucene.2011.10.021. j o u r n a l o f m a t e r i a l s r e s e a r c h a n d t e c h n o l o g y 2 0 2 2 ; 1 8 : 1 0 1 7 e1 0 2 7 [29] Kavaz E, Ekinci N, Tekin HO, Sayyed MI, Aygün B, lu U. Estimation of gamma radiation shielding Peris‚anog qualification of newly developed glasses by using WinXCOM and MCNPX code. Prog Nucl Energy 2019;115:12e20. [30] Tijani SA, Kamal Salahuddin M, Al-Hadeethi Y, Arib Mehenna, Hussein MA, Wageh S, et al. Radiation shielding properties of transparent erbium zinc tellurite glass system determined at medical diagnostic energies. J Alloys Compd 2018;741:293e9. [31] Al-Buriahi MS, Bakhsh Esraa M. Barıs Tonguc, and Sher Bahadar Khan, Mechanical and radiation shielding properties of tellurite glasses doped with ZnO and NiO. Ceram Int 2020;46(11):19078e83. [32] Rammah YS, Olarinoye IO, El-Agawany FI, Mahmoud KA, Akkurt Iskender, Yousef ElSayed. Evaluation of radiation shielding capacity of vanadiumetelluriteeantimonite semiconducting glasses. Opt Mater 2021;114:110897. [33] Kaky Kawa M, Sayyed MI, Ati Ali A, Mhareb MHA, Mahmoud KA, Baki SO, et al. Germanate oxide impacts on the optical and gamma radiation shielding properties of TeO2-ZnO-Li2O glass system. J Non-Cryst Solids 2020;546:120272. [34] Hashim S, Mhareb MHA, Ghoshal SK, Alajerami YSM, Saripan MI, Bradley DA. "Luminescence features of dysprosium and phosphorus oxide co-doped lithium magnesium borate glass. Radiat Phys Chem 2017;137:45e8. [35] Dwaikat Nidal. Analysis of potassium-40 (40K) in soil samples from dhahran city, Saudi Arabia, using gamma spectrometer. Arabian J Sci Eng 2021;46(1):731e5. [36] Ziegler JF, Ziegler MD, Biersack JP. Srim - the stopping and range of ions in matter. Nucl Instrum Methods B 2010;268:1818e23. [37] Sakar E, Ozgür OF, Bünyamin A, Sayyed MI, Kurudirek M. Phy-X/PSD: development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat Phys Chem 2020;166:108496. [38] Ersundu AE, Büyükyıldız M, Çelikbilek Ersundu M, S‚akar E, Kurudirek M. The heavy metal oxide glasses within the WO3 -MoO3 -TeO2 system to investigate the shielding properties of radiation applications. Prog Nucl Energy 2018;104:280e7. https://doi.org/10.1016/j.pnucene.2017.10.008. [39] Kurudirek M. Heavy metal borate glasses: potential use for radiation shielding. J Alloys Compd 2017;727:1227e36. https://doi.org/10.1016/j.jallcom.2017.08.237. [40] Issa Shams AM, Sayyed MI, Zaid MHM, Matori KA. Photon parameters for gamma-rays sensing properties of some oxide of Lanthanides. Results Phys 2018;9:206e10. [41] Al-Ghamdi H, Almuqrin AH, Sayyed MI, Kumar A. The physical, structural and the gamma ray shielding effectiveness of the novel Li2O-K2OeB2O3eTeO2 glasses. Results Phys 2021;29:104726. https://doi.org/10.1016/ j.rinp.2021.104726. [42] Evangelin Teresa P, Naseer KA, Marimuthu K, Alavian H, Sayyed MI. Influence of modifiers on the physical, structural, elastic and radiation shielding competence of Dy3þ ions View publication stats [43] [44] [45] [46] [47] [48] [49] [50] [51] [52] 1027 doped Alkali boro-tellurite glasses. Radiat Phys Chem 2021;189:109741. https://doi.org/10.1016/ j.radphyschem.2021.109741. Divina R, Marimuthu K, Mahmoud KA, Sayyed MI. Physical and structural effect of modifiers on dysprosium ions incorporated boro-tellurite glasses for radiation shielding purposes. Ceram Int 2020;46:17929e37. Sayyed MI, Kumar Ashok, Albarzan Badriah, Jecong JFM, Kurtulus Recep, Almuqrin Aljawhara H, et al. Investigation of the optical, mechanical, and radiation shielding features for strontium-borotellurite glass system: fabrication, characterization, and EPICS2017 computations. Optik 2021;243:167468. Naseer KA, Marimuthu K, Mahmoud KA, Sayyed MI. The concentration impact of Yb3þ on the bismuth borophosphate glasses: physical, structural, optical, elastic, and radiation-shielding properties. Radiat Phys Chem 2021;188:109617. https://doi.org/10.1016/ j.radphyschem.2021.109617. Sathiyapriya G, Naseer KA, Marimuthu K, Kavaz E, Alalawi A, Al-Buriahi MS. Structural, optical and nuclear radiation shielding properties of strontium barium borate glasses doped with dysprosium and niobium. J Mater Sci Mater Electron 2021;32:8570e92. https://doi.org/10.1007/s10854021-05499-0. Naseer KA, Marimuthu K, Mahmoud KA, Sayyed MI. Impact of Bi2O3 modifier concentration on bariumezincborate glasses: physical, structural, elastic, and radiation-shielding properties. Eur. Phys. J. Plus. 2021;136:116. https://doi.org/ 10.1140/epjp/s13360-020-01056-6. Divina R, Naseer KA, Marimuthu K, Alajerami YSM, AlBuriahi MS. Effect of different modifier oxides on the synthesis, structural, optical, and gamma/beta shielding properties of bismuth lead borate glasses doped with europium. J Mater Sci Mater Electron 2020;31:21486e501. https://doi.org/10.1007/s10854-020-04662-3. Chen Q, Naseer KA, Marimuthu K, Kumar PS, Miao B, Mahmoud KA, et al. Influence of modifier oxide on the structural and radiation shielding features of Sm3þ-doped calcium telluro-fluoroborate glass systems. J Australas Ceram Soc 2021;57:275e86. https://doi.org/10.1007/s41779020-00531-8. Naseer KA, Marimuthu K, Al-Buriahi MS, Alalawi A, Tekin HO. Influence of Bi2O3 concentration on bariumtelluro-borate glasses: physical, structural and radiationshielding properties. Ceram Int 2021;47:329e40. https:// doi.org/10.1016/j.ceramint.2020.08.138. Teresa PE, Naseer KA, Piotrowski T, Marimuthu K, Aloraini DA, Almuqrin AH, et al. Optical properties and radiation shielding studies of europium doped modifier reliant multi former glasses. Optik 2021;247:168005. https:// doi.org/10.1016/j.ijleo.2021.168005. Inaba S, Oda S, Morinaga K. Heat capacity of oxide glasses at high temperature region. J Non Cryst Solids 2003;325:258e66. https://doi.org/10.1016/S0022-3093(03)00315-6.