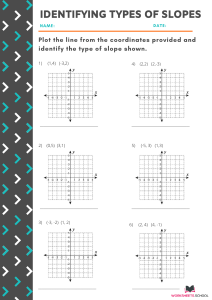
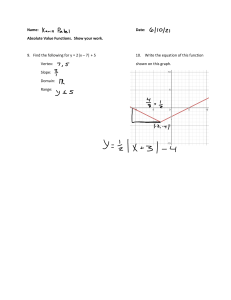
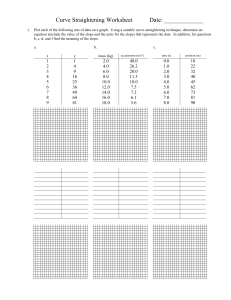
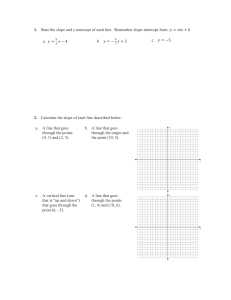
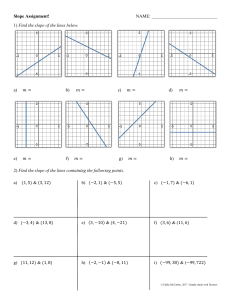
Rates of Reaction By: Spruha, Mahek, Deshna, Jeevika What Is Rate Of Reaction The speed at which a chemical reaction proceeds. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is formed in a unit of time or the concentration of a reactant that is consumed in a unit of time. How to plot a Graph to calculate the Rate of Reaction To plot a graph to calculate the rate of reaction, you need to select two variables, such as time and concentration, and plot them against each other. The slope of the line represents the rate of reaction. Y-Values 18 16 14 12 10 8 6 4 2 0 0 2 4 6 8 10 12 14 16 18 What is the relationship between the calculating rate of reaction and the slope of the graph? The rate of reaction is directly proportional to the slope of the graph. A steeper slope indicates a faster rate of reaction, while a shallower slope indicates a slower rate of reaction. Why use graphs to calculate rates of reaction? Graphs are a useful tool for calculating the rate of reaction because they can visually represent the relationship between variables such as time, concentration, and rate.