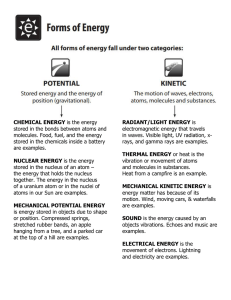
SLIDE 7 Work, energy and power – Work – Energy – Mechanical energy – Internal energy – Interchange of energy between potential energy and kinetic energy – Transfer of energy from one kind to another – Heat energy – Sun as a source of energy explain each with at least 2 examples each 1. Work: Work is the application of force to an object, resulting in the displacement of the object in the direction of the force applied. Mathematically, work (W) is calculated as the product of force (F) and displacement (d) along the direction of the force: W = F * d. Work is measured in joules (J). Examples: a. Lifting a box: When you lift a box from the ground to a shelf, you exert an upward force on the box over a certain distance, doing work against the force of gravity. b. Pushing a car: Pushing a car that has stalled requires applying a force over a distance to move the car and overcome the friction between the tires and the road. 2. Energy: Energy is the ability to do work. It exists in various forms and can be converted from one form to another. The SI unit of energy is also the joule (J). Examples: a. Electrical energy: When you plug in an electronic device like a laptop, electrical energy flows from the power source to the device, allowing it to function. b. Chemical energy: In a battery-powered flashlight, chemical energy is converted into electrical energy when the battery's chemicals undergo reactions, enabling the light to illuminate. 3. Mechanical Energy: Mechanical energy is the sum of potential energy and kinetic energy possessed by an object in motion. It is a form of energy associated with the motion and position of an object. Examples: a. Swinging pendulum: As a pendulum swings, it possesses both kinetic energy (at the lowest point of its swing) and potential energy (at the highest point) due to its position above the ground. b. Rolling ball: A ball rolling down a hill has both kinetic energy (due to its motion) and potential energy (due to its position above the ground). 4. Internal Energy: Internal energy refers to the total energy stored within a system at the microscopic level, considering the kinetic energy of particles and potential energy associated with their positions. It includes both thermal energy (due to the temperature of the system) and the internal potential energy of molecules. Examples: a. Heating water: When you heat a pot of water on a stove, the internal energy of the water increases as the molecules gain kinetic energy, leading to a rise in temperature. b. Compressing a gas: When you compress a gas inside a container, the internal energy of the gas increases due to the increase in kinetic energy of its molecules. 5. Interchange of Energy between Potential Energy and Kinetic Energy: Potential energy is the energy stored in an object due to its position or configuration, while kinetic energy is the energy possessed by an object in motion. Energy can be transferred between these two forms. Examples: a. Falling object: As an object falls, its potential energy decreases while its kinetic energy increases due to its motion under the influence of gravity. b. Bouncing ball: When a ball bounces, it converts its potential energy into kinetic energy as it moves downward, and then back into potential energy as it reaches the highest point of the bounce. formula for kinetic energy: KE = 1/2 * m * v^2 Where: • KE is the kinetic energy of the object (in joules) • m is the mass of the object (in kilograms) • v is the velocity of the object (in meters per second) Math problem: A 50 kg ball is moving at a velocity of 10 m/s. What is the kinetic energy of the ball? Solution: KE = 1/2 * m * v^2 = 1/2 * 50 kg * 10 m/s^2 = 2500 J Therefore, the kinetic energy of the ball is 2500 J. 6. Transfer of Energy from One Kind to Another: Energy can be converted or transferred from one form to another. Examples: a. Solar panels: Solar panels convert sunlight (radiant energy) into electrical energy, which can be used to power various devices. b. Gasoline engine: In a car's gasoline engine, the chemical energy in the fuel is converted into mechanical energy to propel the vehicle. 7. Heat Energy: Heat energy is the transfer of thermal energy from one object or substance to another due to a temperature difference. It flows from hotter to colder regions. Examples: a. Boiling water: As you heat water in a kettle on the stove, heat energy is transferred from the stove's heating element to the water, causing it to boil. b. Radiator heating: In a central heating system, radiators transfer heat energy from hot water or steam to the surrounding air, warming up a room. 8. Sun as a Source of Energy: The sun is a primary source of energy for the Earth, providing a wide range of energy forms essential for life and various processes. Examples: a. Solar energy: Solar panels harness sunlight to generate electricity, as mentioned earlier, making use of photovoltaic cells that convert sunlight into electrical energy. b. Photosynthesis: Plants utilize solar energy to convert carbon dioxide and water into glucose and oxygen through the process of photosynthesis, providing energy for their growth and releasing oxygen into the atmosphere. Greenhouse effect The image shows the following: 1. 2. 3. 4. 5. 6. The sun's rays hit the Earth's atmosphere. Some of the sun's rays are reflected back into space. Some of the sun's rays are absorbed by the Earth's surface. The Earth's surface warms up and emits infrared radiation. Some of the infrared radiation is absorbed by greenhouse gases in the atmosphere. The greenhouse gases trap the heat, which warms the Earth's atmosphere. END OF SLIDE 7 Nuclear energy is the energy released from the nucleus of an atom. It can be released through nuclear fusion or nuclear fission. Nuclear energy is a very powerful form of energy, and it can be used to generate electricity, power ships, and create nuclear weapons. nuclear fusion and nuclear fission:Nuclear fusion is a type of nuclear reaction in which two or more atomic nuclei join together, or "fuse," to form a single heavier nucleus. This is usually accompanied by the release of large quantities of energy. Nuclear fission is the process by which a large nucleus, such as uranium-235, splits into two smaller nuclei, with the release of energy. The top part of the image shows the process of nuclear fusion. Two small atoms, such as hydrogen atoms, fuse together to form a larger atom, such as helium. This process releases a large amount of energy. The bottom part of the image shows the process of nuclear fission. A large atom, such as uranium-235, splits into two smaller atoms, such as krypton-92 and barium-141. This process also releases a large amount of energy.. Here are some additional points that you could include in your exam answer: • Nuclear fusion is the process that powers the sun and other stars. • Nuclear fission is the process that is used in nuclear power plants. • Nuclear energy is a clean and efficient form of energy, but it also has the potential to be dangerous. the process of nuclear power generation, along with a diagram: The diagram shows the basic components of a nuclear power plant. The reactor core is the heart of the plant, and it is where the fission of uranium takes place. The heat generated by the fission is used to boil water, which turns into steam. The steam then turns a turbine, which generates electricity. The electricity is then sent to transformers, which increase the voltage so that it can be distributed to homes and businesses. Explanation: The process of nuclear power generation can be summarized as follows: 1. The fuel (uranium) is placed in the reactor core. 2. The uranium is bombarded with neutrons, which causes it to fission. 3. When uranium fissions, it releases heat. 4. The heat is used to boil water. 5. The steam turns a turbine. 6. The turbine generates electricity. 7. The electricity is sent to transformers. 8. The transformers increase the voltage of the electricity. 9. The electricity is distributed to homes and businesses. The only difference between fossil fuel and nuclear power stations is how the water is heated. In a fossil fuel power station, the water is heated by burning fossil fuels, such as coal or natural gas. In a nuclear power station, the water is heated by the fission of uranium. Nuclear power is a clean and efficient form of energy. It does not produce greenhouse gases, and it is a reliable source of electricity. However, nuclear power also has the potential to be dangerous. If there is a problem with the reactor core, it could lead to a nuclear meltdown. Thermonuclear energy is the energy released from the fusion of atomic nuclei.. It is a very powerful form of energy, and it is the source of energy in stars, including the sun. Thermonuclear fusion is also the process that is used in hydrogen bombs. The process of thermonuclear fusion can be summarized as follows: 1. Two or more atomic nuclei are brought together at very high temperatures and pressures. 2. The nuclei collide and fuse together to form a single heavier nucleus. 3. The fusion of the nuclei releases a large amount of energy. The sun is a good example of thermonuclear fusion. The sun's core is very hot and very dense, and these conditions allow for the fusion of hydrogen nuclei to take place. The fusion of hydrogen nuclei releases a large amount of energy, which is what powers the sun. Hydrogen bombs are also a good example of thermonuclear fusion. Hydrogen bombs are made up of a mixture of deuterium and tritium, which are isotopes of hydrogen. When deuterium and tritium are brought together at very high temperatures and pressures, they fuse together to form helium and release a large amount of energy. Thermonuclear energy is a very powerful form of energy, but it is also very difficult to harness. The temperatures and pressures required for thermonuclear fusion are very high, and it is difficult to maintain these conditions for a long period of time. However, there is ongoing research into the development of thermonuclear fusion reactors, which could provide a clean and abundant source of energy in the future. the influences of nuclear power plant installation on heat rejection, gaseous emissions, environmental impact social impact Heat Rejection Nuclear power plants generate heat as a byproduct of the nuclear reaction. However, some of the heat is rejected to the environment, typically by cooling towers or rivers. This can raise the temperature of the surrounding water, which can have a negative impact on aquatic life. Gaseous Emissions Nuclear power plants do not emit greenhouse gases such as carbon dioxide, but they do emit small amounts of radioactive gases. These gases are released during the operation of the plant, as well as during the mining, enrichment, and fabrication of nuclear fuel. The amount of radioactive gases emitted by a nuclear power plant is very small compared to the amount emitted by fossil fuel power plants. However, there is some concern that these gases could pose a health risk to people living near the plant. Environmental Impact In addition to heat rejection and gaseous emissions, nuclear power plants can also have a negative impact on the environment. The construction of a nuclear power plant can disrupt ecosystems and displace wildlife. The plant itself can also pose a risk of accidents, which could release radioactive materials into the environment. Social Impact The construction and operation of a nuclear power plant can also have a social impact on the surrounding community. The plant can create jobs, but it can also lead to increased traffic and noise pollution. There is also the potential for social unrest, as some people may be opposed to the plant on safety or environmental grounds. The law of conservation of energy states that energy can neither be created nor destroyed; rather, it can only be transformed or transferred from one form to another. This means that the total energy of an isolated system remains constant; it is said to be conserved over time. the diagram shows a roller coaster car moving from the top of a hill to the bottom. The car has potential energy at the top of the hill, and this potential energy is converted to kinetic energy as the car moves down the hill. The kinetic energy of the car is then converted back to potential energy as the car reaches the bottom of the hill. Horsepower is a unit of measurement for power, and it is defined as the amount of work that a horse can do in one minute. The text in the image tells us that 1 horsepower is equal to 550 foot-pounds per second. This means that a horse can do 550 foot- pounds of work in one second. END OF SLIDE 8 Atoms are the smallest units of matter that can exist. They are made up of three subatomic particles: protons, neutrons, and electrons. Protons and neutrons are found in the nucleus of the atom, while electrons orbit the nucleus. Molecules are groups of two or more atoms that are chemically bonded together. The atoms in a molecule can be of the same element, or they can be of different elements. For example, a molecule of water is made up of two hydrogen atoms and one oxygen atom. Here are some examples of atoms and molecules: • Atoms: Hydrogen, oxygen, carbon, nitrogen, etc. • Molecules: Water, carbon dioxide, methane, ammonia, etc. • Here are some of the properties of atoms and molecules: Mass: The mass of an atom is determined by the number of protons and neutrons in its nucleus. Charge: Atoms can be neutral, positively charged, or negatively charged. The charge of an atom is determined by the number of protons and electrons it has. Brownian motion Brownian motion is the random motion of particles suspended in a fluid. These dust particles move in a haphazard way because they are constantly hit by the fast moving particles of air. Smoke particles in air: If you look at a plume of smoke in the air, you will see that the smoke particles are moving in a random, zigzag pattern. This is also Brownian motion. The Kinetic Theory of Matter states that matter is composed of a large number of small particles—individual atoms or molecules—that are in constant motion. The kinetic theory of matter can be used to explain the properties of matter in different phases. For example, the particles in a solid are tightly packed together and have very little kinetic energy. This is why solids have a fixed shape and volume. The particles in a liquid are more loosely packed together and have more kinetic energy. This is why liquids can flow and take the shape of their container. The particles in a gas are very spread out and have a lot of kinetic energy. This is why gases can expand to fill their container. Adhesion and cohesion • Adhesion is the force of attraction between the molecules of two different substances. For example, water molecules are attracted to the molecules of glass, which is why water can "stick" to a glass surface. • Cohesion is the force of attraction between the molecules of the same substance. For example, water molecules are attracted to each other, which is why water forms droplets. Here are some examples of adhesion and cohesion in action: • Water droplets: Water droplets form because of the cohesive forces between water molecules. The water molecules are attracted to each other more than they are to the air molecules, so they form small spheres. • Capillary action: Capillary action is the ability of water to rise up through a narrow tube. This is due to the adhesion of water molecules to the walls of the tube. The water molecules are attracted to the walls of the tube more than they are to each other, so they climb up the walls of the tube. surface tension The cohesive forces between molecules in a liquid are shared with all neighbouring molecules. Those on the surface have no neighbouring molecules above and, thus, exhibit stronger attractive forces upon their nearest neighbours on and below the surface. Surface tension could be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water molecules. how surface tension allows a water strider to "walk on water".? Surface tension is the force that acts at the surface of a liquid, causing it to behave as if it has a thin skin. This is due to the cohesive forces between the liquid molecules. The water strider's legs are covered in tiny hairs that are hydrophobic, meaning that they repel water. This means that the water molecules are unable to wet the hairs, and the surface tension of the water is able to support the weight of the water strider. The water strider's legs are also very thin, which helps to distribute its weight over a large surface area. This further reduces the chances of the water strider breaking the surface tension of the water. In addition, the water strider's legs are constantly moving, which helps to break up the surface tension of the water and prevent it from forming a film around the legs. As a result of these factors, the water strider is able to walk on the surface of water without sinking. osmosis osmosis is the movement of water molecules from an area of high water concentration to an area of low water concentration through a semi-permeable membrane. A semi-permeable membrane is a barrier that allows some molecules to pass through, but not others. In the case of osmosis, the membrane allows water molecules to pass through, but it does not allow other molecules, such as solutes, to pass through. The movement of water molecules through a semi-permeable membrane is driven by the difference in water concentration on the two sides of the membrane. The side with the higher water concentration is called the hypotonic solution, and the side with the lower water concentration is called the hypertonic solution. Water molecules will move from the hypotonic solution to the hypertonic solution until the water concentrations on the two sides of the membrane are equal. This process is called osmosis. Elasticity is the property of solid materials to return to their original shape and size after the forces deforming them have been removed. The greater the resistance to change, the greater is the elasticity of the material and the faster it comes back to its original shape or configuration when the deforming force is removed. So iron is more elastic than rubber END OF SLIDE 9 Today’s Discussion Points – Hooks law – Fluid friction Atoms – the Big Idea - Atoms, elements and compounds - Atomic structure: different models - How electrons are arranged Hooke's law Hooke's law states that the force required to extend or compress a spring is proportional to the amount of stretch or compression. Hooke's Law can be summarized as "the more you stretch or compress a spring, the more force it will exert back in the opposite direction." Mathematically, Hooke's Law is expressed as: F = -k * x Where: F is the force applied to the spring or elastic material, k is the spring constant (a measure of the stiffness of the spring or material), and x is the amount of deformation (the change in length) of the spring. the spring constant is a measure of the stiffness of the spring. A spring with a high spring constant is more difficult to stretch or compress than a spring with a low spring constant. Here are some examples of Hooke's law: • When you stretch a rubber band, the force required to stretch it is proportional to the amount of stretch. The greater the stretch, the greater the force required. When you compress a spring, the force required to compress it is proportional to the amount of compression. The greater the compression, the greater the force required. 1. Atoms and Force: Now, let's dive a little deeper. All objects are made up of tiny building blocks called atoms. When you stretch or compress an object, these atoms move from their normal positions. Hooke's Law tells us that the amount of these tiny movements is directly related to the force we apply. If we push or pull lightly, the atoms move only a little bit. If we push or pull harder, the atoms move more. Hooke's Law works best for materials that are elastic, like rubber or 2. certain Elastic materials can handle these tiny atomic movements and return to Elastic metals. Materials: their original shape once the force is gone. It's like a "bounce-back" effect: they deform under the force, but they want to get back to their original arrangement when the force is removed. • What is fluid friction? The resistance to an object’s motion in a fluid is called fluid friction. However, the resistance is not restricted to solid objects only. It also occurs within the different layers of the fluid, which can be liquid or gas. When it occurs within the fluid, the friction of fluid flow is called viscosity. A high viscosity fluid will be more viscous than a low viscosity fluid. For example, honey is more viscous than water and does not flow as smoothly as water. the amount of fluid friction depends on a number of factors, including the viscosity of the fluid, the speed of the fluid, and the surface roughness of the two surfaces. • Viscosity: Viscosity is a measure of how much a fluid resists flow. The higher the viscosity, the more fluid friction there will be • Speed: The faster the fluid is moving, the more fluid friction there will be. • Surface roughness: The rougher the surface, the more fluid friction there will be. • The design of ships and airplanes. The shape of a ship or airplane is designed to reduce fluid friction, which helps to improve fuel efficiency. Here are some examples of fluid friction: • When you swim, you feel the resistance of the water against your body. This is fluid friction. • When you drive a car, you feel the drag of the air against the car. This is also fluid friction. • When you pour a glass of water, the water flows slowly down the side of the glass. This is because of the fluid friction between the water and the glass. Property Compound Element Definition Substance made up of two or more different elements that are chemically bonded together Substance that cannot be broken down into simpler substances by chemical means Examples Water (H2O), table salt (NaCl), carbon dioxide (CO2) Hydrogen (H), oxygen (O), gold (Au) Molecule Smallest unit of a compound that can exist independently Water molecule (H2O), carbon dioxide molecule (CO2) atomic structure • Atoms are the basic unit of matter. They are made up of three types of subatomic particles: protons, neutrons, and electrons. • Protons and neutrons are found in the nucleus of the atom. The nucleus is very small, but it contains most of the atom's mass. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. • Electrons orbit the nucleus in shells. The shells are arranged in concentric circles, and each shell can hold a certain number of electrons. The outermost shell is called the valence shell, and it is the shell that determines the chemical properties of the atom. • The number of protons in an atom's nucleus is called the atomic number. The atomic number determines the element of the atom. For example, all atoms with 6 protons are carbon atoms. The number of neutrons in an atom's nucleus can vary. Atoms with the same number of protons but different numbers of neutrons are called isotopes. Atoms of the same element with different numbers of neutrons but same number of protons numbers) are called isotopes of that (and hence different mass • element. For example, carbon-12 has 6 protons and 6 neutrons, while carbon-14 has 6 protons and 8 neutrons. The nucleus is at the center of the atom, and it is surrounded by the electron shells. The electrons are represented as circles, and the number of electrons in each shell is shown. Energy shells Electrons are arranged in different shells around the nucleus. The innermost shell - or lowest energy shell - is filled first. Each succeeding shell can only hold a certain number of electrons before it becomes full. The innermost shell can hold a maximum of two electrons, the second shell a maximum of eight, and so on. Rutherford's model of the atom: Rutherford's model is a model of the atom that was proposed by Ernest Rutherford in 1911. It is based on his famous gold foil experiment, in which he showed that the atom is mostly empty space, with a small, dense nucleus at the center. The nucleus of the atom contains the protons and neutrons, which make up most of the atom's mass. The electrons orbit the nucleus in shells, much like planets orbiting the sun. The electrons are negatively charged, while the nucleus is positively charged. This means that the electrons are attracted to the nucleus, but they are also moving very fast, which keeps them from being pulled into the nucleus. The Rutherford model is often represented as a solar system, with the nucleus as the sun and the electrons as the planets. However, it is important to remember that the electrons do not orbit the nucleus in the same way that planets orbit the sun. The electrons are actually spread out in a cloud around the nucleus, and they can occupy different energy levels. Here are some examples of how Rutherford's model of the atom is used today: • Chemistry: Rutherford's model is used to explain the chemical properties of atoms. For example, the number of electrons in the outermost shell of an atom determines how that atom will react with other atoms. Bohr's model of atom here's the key idea of Bohr's model: Electrons can only exist in specific energy levels or orbits around the nucleus, just like planets can only be at certain distances from the sun. These energy levels are also called "shells" or "electron shells." When an electron is in its lowest energy level (closest to the nucleus), it's in its most stable state, and we call this the ground state. But, if the electron gains some energy (by absorbing light or heat, for example), it can jump to a higher energy level, farther from the nucleus. This is called an "excited state." However, the electron can't stay in this excited state forever. It's like a planet being flung farther from the sun; eventually, it will lose that extra energy and fall back to a lower energy level. When it does that, it releases the extra energy it gained in the form of light. This is what gives us the beautiful colors in fireworks, neon lights, and other glowing objects. Here's an example: Think of a neon sign. Inside the glass tube, there is neon gas. When electricity passes through the gas, it excites the electrons in the neon atoms, making them jump to higher energy levels. But they can't stay there, so when they fall back to lower levels, they emit light in the visible range, giving us the bright, colorful glow we see in the sign END OF SLIDE 10 Prepared by Nayan Paul