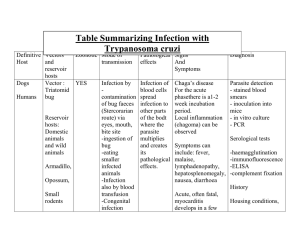
Microbiology and parasitology Final lecture Protozoa Definition of terms: Infective stage – refers to the stage of the parasite that enters the host or the stage that is present in the parasite’s source of infection. Pathogenic stage – refers to the stage of the parasite that is responsible for producing the organ damage in the host leading to the clinical manifestations. Encystation – process by which trophozoites differentiate into cyst forms. Excystation – process by which cysts differentiate into trophozoite forms General Properties of Protozoa The Kingdom Protozoa consists of single celled eukaryotic organisms that are spherical to oval or elongated in shape. classification of these organisms is mainly based on the organ of locomotion utilized. Some are facultative parasites capable of a free-living state (e.g., Acanthamoeba and Naegleria). - normally reside in the soil or water but can cause severe illness when they gain entrance into the central nervous system or the eyes. Majority of protozoa divide by means of binary fission (flagellates, ciliates, and amebae). Sporozoans reproduce through both sexual and asexual means. - Asexual reproduction is achieved through a process called merogony or schizogony. - Sexual recombination can occur, leading to antigenic and genomic variation. They are small in size Protozoan infections are most often diagnosed through microscopic examination of body fluids, tissue specimens, or feces. Special stains may be used to demonstrate the different protozoa. Parasitic protozoa infections are diagnosed by demonstrating the motile, feeding, dividing stage of the parasite called trophozoite, or - The trophozoite is the motile (with pseudopods or “false feet”) and feeding form and is the pathogenic stage. The dormant, non-motile form called the cyst. - The cyst is the non-motile form and is the infective stage for most intestinal protozoan parasites, except for - Trichomonas vaginalis where cyst forms are not found. Intestinal and Urogenital Protozoa Subphylum Sarcodina: Entamoeba histolytica Important properties and life cycle An intestinal and tissue ameba and is the only known pathogenic intestinal ameba Life cycle consists of two stages - the non-motile cyst (infective stage) and - the motile trophozoite (pathogenic stage). The trophozoite is found within the intestinal and extra intestinal lesions, and in diarrheal stools. Cysts are usually found in nondiarrheal, formed stools. development of abscess in the liver. Epidemiology and Pathogenesis Entamoeba histolytica is found worldwide but is more common in tropical countries, especially in areas with poor sanitation. Primarily transmitted by the fecal oral route through ingestion of the cyst from contaminated food and water. Water serves as the major source of infection of the parasite. Sexual transmission may also occur in unprotected sex with a woman who has vaginal amoebiasis or through anal intercourse. Ingested cyst undergoes excystation in the ileum where it differentiates into a trophozoite (pathogenic stage) proceeds to colonize the cecum and colon. Trophozoites undergo encystation and become converted into cysts, which are then passed out with the feces. - Trophozoites are usually recovered in the feces of patients with active infection (diarrheic stools) while cysts are found in formed, non-diarrheic stools. - Trophozoites of E. histolytica secrete enzymes that cause local necrosis producing the typical “flask shaped” ulcer associated with the parasite. Invasion of the portal circulation may occur leading to the Once we going to ingested the infective cyst and we get it from the contaminated food or water. Once ingested this parasite will take residency or find a habitat either in the intestinal tract or in the liver. And that infective stage will transform into pathogenic stage. From cyst they will then form into a trophozoites. The trophozoites is what we consider the motile or pathogenic stage clinical signs and symptoms will be manifested here. This is only a cycle any individual infected with this kind of protozoa. Once they pass out a urine especially due to no proper disposal very common on slum areas, they can pass the infection to other individuals. From a cyst which is the infective stage once they will find a phylum especially in our gastrointestinal tract it will transform into trophozoite and this is the pathogenic stage which we are going to experience the clinical signs and symptoms especially the diarrhea. It can even migrate to other organs such as our liver and it can cause a hepatic abscess or abnormal condition of the liver due to the toxin produce by these protozoa. Disease: Amoebiasis 1. Acute intestinal amoebiasis - presents as bloody, mucus containing diarrhea (dysentery) accompanied by lower abdominal discomfort, flatulence (release of gas), and tenesmus (feeling of incomplete defecation). - Chronic infection may occur, with symptoms such as occasional diarrhea, weight loss, and fatigue. - Lesion called an amoeboma may form in the cecum or in the rectosigmoid area of the colon, which may be mistaken for a malignant tumor in the colon. 2. Extraintestinal amoebiasis - Occurs when the parasite enters the circulatory system. - Most common extraintestinal form of amoebiasis is the amoebic liver abscess. Characterized by right upper quadrant pain, weight loss, fever, and a tender, enlarged liver. - Abscess found on the right lobe of the liver may penetrate the diaphragm and cause lung disease (amoebic pneumonitis). - Other organs that may become infected include the pericardium, spleen, skin, and brain (meningoencephalitis). 3. Asymptomatic carrier state - Occurs under the following conditions: (a) if the parasite involved is a low virulence strain; (b) if the parasite load is low; and (c) if the patient’s immune system is intact - The patient presents with no symptoms but the parasite reproduces and is passed out with the patient’s feces. Laboratory Diagnosis Intestinal amoebiasis is confirmed by the finding of trophozoites in diarrheic stools or cysts in formed stools. Trophozoites characteristically contain ingested red blood cells. Stool specimen is examined within one hour of collection to see the motility of the trophozoites. Serologic testing may be useful for the diagnosis of invasive amoebiasis. Treatment Metronidazole - for symptomatic intestinal amoebiasis or hepatic abscess Alternative drug tinidazole - for both intestinal and extraintestinal amoebiasis. Diloxanide furoate, metronidazole, or paromomycin – for asymptomatic carriers. Surgical drainage of amoebic liver abscess may be necessary if there is no improvement with medical therapy. Prevention and Control Most important preventive measure observance of good personal hygiene. This includes: 1. Proper hand washing, especially for food handlers. 2. Proper waste disposal should be observed to avoid fecal contamination of water sources. 3. Use of “night soil” (human feces) for fertilization of crops must be avoided. 4. Adequate washing and cooking of vegetables should be observed. Subphylum Mastigophora:Giardia lamblia (Giardia intestinalis) Important Properties and Life Cycle Giardia lamblia is an intestinal protozoan that was initially known as Cercomonas intestinalis. Another name used is Giardia duodenale. Also exists in a cyst form and a trophozoite form. Trophozoite is pear shaped or teardrop shaped with four pairs of flagella and has a motility likened to a falling leaf. Described as resembling an old man with whiskers (“old man facies”). Possesses a sucking disc which the parasite uses to attach itself to the intestinal villi of the infected human. Cyst is typically oval and thick walled with four nuclei. The fully mature cyst contains four nuclei with four median bodies. It divides through binary fission. Each cyst gives rise to two trophozoites during excystation in the intestinal tract. Epidemiology and pathogenesis Giardia lamblia has a worldwide distribution through contaminated water sources. Disease can occur in outbreaks related to contaminated water supplies 50% of infected individuals do not present with symptoms and serve as carriers. Other than humans, many species of mammals may act as reservoirs. Infection is also common among individuals engaging in oral anal contact. High incidence has been seen in daycare centers and among patients in mental hospitals. Life cycle The parasite is transmitted through ingestion of the cyst from fecally contaminated water and food. Cyst enters the stomach and is stimulated by the gastric acid to undergo excystation in the duodenum. Trophozoites attach themselves to the duodenal mucosa through the sucking disks. Damage to the intestines is not due to invasion of the parasite but because of inflammation of the duodenal mucosa, leading to diarrhea with malabsorption of fat and proteins. Trophozoites may also infect the common bile duct and gallbladder. These may include deficiencies in fat soluble vitamins, folic acid, and proteins. - A self-limiting infection, lasting one to two weeks. Relapses may occur, especially in patients with IgA deficiency. Laboratory diagnosis The demonstration of the cyst or trophozoite (or both) in diarrheic stools. Only cysts are isolated from the stools of asymptomatic carriers. If microscopic examination of the stool is negative, string test may be performed which consists of making the patient swallow a weighted piece of string until it reaches the duodenum. Trophozoites adhere to the string and can be visualized after withdrawal of the string. Treatment Metronidazole, tinidazole, and nitazoxanide - primary choice of treatments for G. lamblia infection. As per recommendation of the Centers for Disease Control and Prevention in the United States, Prevention and control 1. Avoidance of fecal contamination of water supplies through proper waste disposal. 2. Drinking water should be boiled, filtered, or iodine treated especially in endemic areas. 3. Proper hand washing is also recommended. - Disease: Giardiasis 1. Asymptomatic carrier state - Infection with the parasite is usually completely asymptomatic. - Infected individual unknowingly passes out the parasite with the feces which can then contaminate water. 2. Giardiasis (Traveler’s diarrhea) - infection is characterized by a non-bloody, foul smelling diarrhea accompanied by nausea, loss of appetite, flatulence, and abdominal cramps. - The symptoms may persist for weeks or months. - Malabsorption of fat may lead to the presence of fat in the stool (steatorrhea). - Patients are usually afebrile. Manifestations may vary depending on which nutrient becomes deficient due to the resulting malabsorption. Subphylum Mastigophora: Trichomonas vaginalis Important properties and Life cycle The parasite is a pearshaped organism with a central nucleus, four anterior flagella, and an undulating membrane. exists only in the trophozoite form (infective and pathogenic). Epidemiology and pathogenesis Trichomonas vaginalis is not an intestinal pathogen. Causes urogenital infections The main mode of transmission is through sexual intercourse. isolated from the urethra and vagina of infected women as well as the urethra and prostate gland of infected men. Infection is highest among sexually active women in their thirties. Lowest in postmenopausal women. Parasite may be transmitted through toilet articles and clothing of infected individuals. Infants may be infected as they pass through the infected birth canal during delivery. Parasite invades the vaginal mucosa of infected women. They multiply through binary fission. Trophozoites feed on local bacteria and leukocytes. In men, the most common infection site is the prostate gland and the urethral epithelium. Disease: Trichomoniasis Infection in men - Usually asymptomatic and men serve as the reservoir for infection in women. - In men who develop symptoms, the manifestations are those related to development of prostatitis (inflammation of the prostate), urethritis (manifest as discharge), and other urinary tract involvement. - Persistent or recurring urethritis is the most common symptomatic form of the infection. Infection in women - Also asymptomatic, some women may present with scant, watery vaginal discharge. - In more severe cases, the discharge may be foul smelling and greenish yellow in color. - May be accompanied by itching (pruritus) and a burning sensation in the vagina. - The cervix appears very red, with small punctuate hemorrhages, giving rise to a strawberry cervix. - Other common symptoms include dysuria and increased frequency of urination. Infection in infants - occurs as the infant passes through the infected birth canal of the mother during vaginal delivery. - The infected infants may manifest conjunctivitis or respiratory infection. Laboratory Diagnosis Diagnosis is made by the finding of the characteristic trophozoite in a wet mount of vaginal or prostatic secretions, urine, and urethral discharges. Treatment Metronidazole - The drug of choice for treatment of trichomoniasis. All sexual partners of an individual with the infection must be simultaneously treated to prevent “ping pong” infections. Prevention and Control 1. Practice safe sex 2. Use of condoms can limit the transmission of the parasite. 3. Health and sex education are important 4. Maintenance of the acidic pH of the vagina may also be helpful. Phylum Ciliophora: Balantidium coli Important Properties and Life cycle Balantidium coli is morphologically more complex than E. histolytica. It has a primitive mouth called a cytostome, a nucleus, food vacuoles, and a pair of contractile vacuoles. The infective stage: cyst The pathogenic stage: trophozoite Invades the mucosal lining of the terminal ileum, cecum, and colon. It is the largest protozoan to infect humans. The trophozoites typically exhibit: - Rotary - Boring motility (through cilia) - contain two nuclei (a small dot like micronucleus adjacent to a kidney bean shaped macronucleus). The cyst also contains two nuclei although the micronucleus may not be readily observable. Epidemiology and pathogenesis Parasite has a worldwide distribution Most common and most important reservoir is the pig. Monkeys may occasionally act as reservoirs of the parasite. Main source of infection is water contaminated by pig feces and the mode of transmission is through the fecal oral route. Person to person transmission via food handlers has been implicated in outbreaks. Cysts are found in contaminated water, which when ingested, undergoes excystation in the small intestines. The trophozoites travel to the large intestines where they produce ulcers similar to those seen in amoebiasis. However, extra intestinal involvement is not seen. Disease: Balantidiasis Most infected individuals are asymptomatic A dysenteric type of diarrhea resembling amebic dysentery may occur in patients with high parasite load. Acute infections manifest with liquid stools containing pus, blood, and mucus. Chronic infections may manifest with a tender colon, anemia, wasting (cachexia), and alternating diarrhea and constipation. Extraintestinal infection is rare and may involve the liver, lungs, mesenteric nodes, and urogenital tract. Laboratory Diagnosis Diagnosis is based on the finding of trophozoites and cysts in the stool specimen. Due to its large size, the parasite can be readily detected in fresh, wet microscopic preparations. Treatment Oxytetracycline and iodoquinol - The current recommended treatment of patients with balantidiasis Metronidazole - may also be used as alternative to treat infected patients. Prevention and Control 1. Maintenance of sanitary hygiene, proper disposal of pig feces. 2. Boiling of drinking water. Blood and Tissue Protozoa Subphylum Sarcodina: Acanthamoeba (Free living Amoeba) Important Properties and Life cycle Acanthamoeba Castellani, together with Naegleria, is a minor protozoan pathogen but unlike Naegleria, Acanthamoeba usually causes infection in immunocompromised patients A free-living amoeba that causes inflammation of the brain substance and its meningeal coverings (meningoencephalitis). Parasite is found widely in soil, contaminated freshwater lakes, and other water environment. Able to survive in cold water. Like E. histolytica, Infective stage: is the cyst Pathogenic stage: the trophozoite. Epidemiology and pathogenesis Two ways by which the parasite can be acquired - Through aspiration or nasal inhalation. - Through direct invasion in the eye. People acquire the infection usually while swimming in contaminated water. Inhalation of the cysts from dust has also been shown to occur. The trophozoites enter through the lower respiratory tract or through ulcers in the mucosa or skin. Parasite then migrates through the bloodstream and invade the central nervous system. Eye infection with Acanthamoeba occurs primarily in patients who wear contact lenses. Tap water contaminated with the parasite is the source of infection for contact lens users. Disease 1. Granulomatous amebic encephalitis - infection occurs primarily in immunocompromised individuals. - The parasite produces a granulomatous amebic encephalitis and brain abscesses in immunocompromised patients. - Symptoms develop slowly and may include headache, seizures, stiff neck, nausea, and vomiting. - The brain lesions may contain both the trophozoites and the cysts. - In rare instances, the parasite may spread and produce granulomatous lesions in the kidneys, pancreas, prostate, and uterus. 2. Keratitis - infection of the cornea of the eye. - Symptoms include severe eye pain and vision problems. - Loss of vision may occur due to perforation of the cornea. Laboratory Diagnosis By finding of both trophozoites and cysts in the cerebrospinal fluid as well as brain tissue and corneal scrapings. Histologic examination of corneal scrapings may also be done. Calcofluor white, a stain usually used to demonstrate fungi, may be used to demonstrate the parasite in corneal scraping specimens. Treatment Pentamidine, Ketoconazole, or Flucytosine - effective in the treatment of infection, however, prognosis is poor even with treatment. Topical miconazole, chlorhexidine, itraconazole, ketoconazole, rifampicin, or propamidine - for eye and skin involvement. Propamidine - has been documented to have the best success record. Prevention and Control 1. Adequate boiling of water. 2. Regular disinfection of contact lenses is also advised. 3. Contact lens wearers are also advised to avoid using homemade non sterile saline solutions. Subphylum Sarcodina: Naegleria Important Properties and Life Cycle Similar to Acanthamoeba, the parasite Naegleria is also classified as a freeliving protozoan. It shares many characteristics with Acanthamoeba. Parasite is also found worldwide in soil and contaminated water environment. Naegleria can survive in thermal spring water. The known pathogen worldwide is Naegleria fowleri, which is the only amoeba with three identified morphologic forms - trophozoite, - flagellate, - and cyst forms. The trophozoite exhibits the typical amoeboid motility which is described as “slug like.” Flagellate form is pear shaped and is equipped with two flagella that is responsible for the parasite’s jerky or spinning movement. The non motile form is the cyst Amoeboid trophozoite form is however the only form that is known to exist in humans. Epidemiology and pathogenesis Naegleria infection is usually acquired trans nasally when swimming in contaminated water. Parasite penetrates the nasal mucosa and cribriform plate, enters the central nervous system, and produces a rapidly fatal meningitis and encephalitis (primary amoebic meningoencephalitis. The parasite produces infection in otherwise healthy individuals, usually children. The parasite may be acquired through inhalation of dust containing the parasite. Entire life cycle of the parasite (amoeboid trophozoite → flagellate trophozoite → amoeboid trophozoite → cyst form) occurs entirely in the external environment. Disease 1. Asymptomatic infection – the most common clinical presentation in patients with colonization of the nasal passages. 2. Primary amoebic meningoencephalitis (PAM) – the result of colonization of the brain by the amoeboid trophozoites leading to rapid tissue destruction. Patients initially complain of sore throat, nausea, vomiting, fever, and headache. Patients eventually develop signs of meningeal irritation (e.g., Kernig’s sign) as well as alterations in their senses of smell and taste. If untreated, the patients may die within one week after onset of symptoms. Laboratory Diagnosis Diagnosis is based on the finding of the amoeboid trophozoites in the cerebrospinal fluid. Treatment Treatment is ineffective because of its rapidly fatal course. However, some patients have been shown to recover from infection due to early detection and initiation of treatment. Treatment of choice is Amphotericin B in combination with miconazole and rifampicin (Murray, 2014). Prevention and Control There is no known means of preventing Naegleria infection other than the prevention of contamination of water sources. Adequate chlorination of swimming pools and hot tubs is recommended. Subphylum Mastigophora: Hemoflagellates Leishmania spp. Important Properties and Life Cycle The life cycle of the parasite involves a vector, the female sandfly of the Phlebotomus and Lutzomyia genera. Leishmania specie. are obligate intracellular parasites. It has three morphologic forms 1. the amastigote 2. promastigote and 3. epimastigote. The infective stage is the promastigote. The promastigote form may be seen only if a blood sample is collected and examined immediately after transmission. Epimastigotes are found primarily in the vector. The pathogenic stage and diagnostic form is the amastigote which is found primarily in tissue and muscle, as well as the central nervous system within macrophages and in cells of the reticuloendothelial system. The amastigote is: - Round to oval in shape and contains a nucleus - A basal body structure called a blepharoblast - a small parabasal body located adjacent to the blepharoblast. - Both the blepharoblast and parabasal body are collectively known as the kinetoplast. The promastigote is: - Long and slender with a kinetoplast located in its anterior end - a single free flagellum extending from the anterior portion. Epidemiology and Pathogenesis The parasite has a worldwide distribution. Natural reservoirs include rodents, ant eaters, dogs, and cats. In endemic areas, the parasite may be transmitted in a human vector human cycle. There are three major strains of Leishmania which differ in the tissues affected and the resulting clinical manifestations. These are: - Leishmania donovani (visceral leishmaniasis) - Leishmania tropica (cutaneous leishmaniasis) - Leishmania braziliensis (mucocutaneous leishmaniasis). Leishmania donovani complex L. donovani is the causative agent of visceral leishmaniasis (also known as kala azar or dumdum fever). The complex consists of: 1. L. donovani chagasi which is mainly seen in Central America (mainly Mexico, West Indies, and South America) Transmitted by the Lutzomyia sandfly; 2. L. donovani found in parts of Africa and Asia (Thailand, India, China, Burma, and East Pakistan) and is transmitted by the Phlebotomus sandfly; 3. L. donovani infantum, also transmitted by the Phlebotomus sandfly and is found mainly in Mediterranean Europe, Near East, and Africa. The promastigote is injected into the human host through bite of the sand fly. After entry into the host, it loses its flagella, is engulfed by macrophages, and transforms into amastigotes. The organs of the reticuloendothelial system (liver, spleen, and bone marrow) are the most severely affected. Disease: Visceral Leishmaniasis(Kala azar, Dumdum Fever) After an incubation period of 2 weeks to 18 months, the disease begins with intermittent: - Fever - Weakness - weight loss. Massive enlargement of the spleen (splenomegaly) is characteristic, leading to: - hypersplenism and resulting anemia. - Hepatomegaly or enlargement of the liver - In light skinned patients, hyperpigmentation of the skin may be seen (kala azar means “black sickness” or “black fever”). Involvement of the bone marrow leads to : - destruction of the cellular components with the corresponding clinical effect. - anemia due to destruction of red blood cells - bleeding tendencies due to reduction of platelets (thrombocytopenia) - increased risk for secondary infection because of reduction of white blood cell (leukopenia). - Glomerulonephritis or inflammation of the glomeruli of the kidney The disease may be fatal if untreated. Laboratory Diagnosis The screening test is called the Montenegro skin test. This test is similar to the tuberculin skin test for the diagnosis of tuberculosis. It is used as screening for large populations at risk but is not used for diagnosis. Definitive diagnosis is done by demonstration of the amastigote from Giemsa-stained slides of specimen from blood, bone marrow, lymph nodes, and biopsies of infected areas. Culture of blood, bone marrow, and other tissues may also be done, which will show the promastigote forms. Serologic tests are now also available such as indirect fluorescent antibody (IFA) Enzyme linked immunosorbent assay (ELISA), or direct agglutination test (DAT). Treatment liposomal amphotericin B (Ambisome) - The present recommended drug of choice Sodium stibogluconate has also been found to be effective but the development of resistance may occur. gamma interferon in combination with pentavalent antimony - Other patients have shown favorable responses. Prevention and Control 1. The use of insect repellents 2. protective clothing 3. installation of screens may be helpful. 4. Prompt treatment of infected humans is essential to help halt the spread of the disease. Leishmania braziliensis complex L. braziliensis is the causative agent of mucocutaneous leishmaniasis which involves skin, cartilage, and mucous membranes. Infection with L. braziliensis occurs most commonly in Brazil and Central America, primarily in construction and forestry workers. The complex consists of: - L. panamensis (Panama and Colombia), L. peruviana (Peruvian Andes), and - L. guyanensis (The Guianas, parts of Brazil and Venezuela). Infection is transmitted by sandflies (Lutzomyia and Psychodopigus) through skin bite. The promastigotes invade the reticuloendothelial cells where they transform into amastigotes (diagnostic stage). Reproduction of the amastigotes result in tissue destruction. The amastigotes are taken up by the vector during a blood meal and are transformed into promastigotes. Disease: Mucocutaneous Leishmaniasis Mucocutaneous leishmaniasis, also called espundia, begins with a papule at the site of insect bite, then forms metastatic lesions, usually at the mucocutaneous junction of the nose and mouth. Disfiguring granulomatous, ulcerating lesions destroy the nasal cartilage (tapir nose) but not the adjacent bone. Death can occur from secondary infections. Laboratory Diagnosis Diagnosis is confirmed by demonstration of amastigotes in clinical specimen. - Ulcer biopsy specimens are used for the diagnosis of mucocutaneous leishmaniasis. - Microscopic examination of Giemsa stained ulcer biopsy specimens reveals the diagnostic amastigotes. - Culture of infected material may show the promastigotes. - Serologic testing may also be done - Treatment Sodium stibogluconate - the most widely used drug for the treatment of mucocutaneous leishmaniasis Due to resistance has been shown to develop. Alternative drugs include - liposomal Amphotericin B - oral anti fungal drugs (fluconazole, ketoconazole, and itraconazole). Prevention and Control 1. The most important preventive measure is the control of the insect vector. If this cannot be done, measures should be undertaken to protect individuals from sandfly bites 2. Using netting 3. Window screens 4. Protective clothing 5. Insect repellents. 6. Prompt treatment can also help prevent spread of the disease. Leishmania tropica complex Important Properties and Life Cycle The complex consists of L. tropica, L. aethiopica, and L. major. These are the causative agents of what is referred to as Old World cutaneous leishmaniasis. The life cycle of L. tropica is similar to that of L. braziliensis. All three members of the complex are transmitted by the Phlebotomus sandfly and primarily attacks the human lymphoid tissue of the skin. Disease: Old World Cutaneous Leishmaniasis The disease is also known as oriental sore, and Baghdad or Delhi boil. It is characterized by one or several pus containing ulcers that may heal spontaneously. The initial lesion is a small, pruritic red papule at the bite site. In patients with anergy and hypersensitivity responses, spontaneous healing does not occur. Thick skin plaques with multiple nodules may develop, especially on the limbs and face. Laboratory Diagnosis Microscopic examination of Giemsa stained slides of fluid aspirated from beneath the ulcer bed is the usual diagnostic procedure of choice. Microscopic examination reveals the typical amastigotes. Culture of specimen will show the promastigote form. Serologic tests are also available. Treatment The drug of choice is sodium stibogluconate. Steroids with application of heat to the infected lesions may be used. Other alternative drugs are meglumine antimonite, pentamidine, and oral ketoconazole. Paromomycin ointment may be helpful in the healing of the ulcers. Prevention and Control 1. Preventive measures are the same as those for the different forms of leishmaniasis. 2. However, unlike the other Leishmania, a vaccine has been developed against L. tropica which is currently undergoing clinical trials. Trypanosoma spp. Important Properties and Life Cycle The trypanosomes are also hemoflagellates like Leishmania. The major difference between the two lies in their diagnostic stages – - Which is the amastigote for Leishmania - the trypomastigote for the trypanosomes. The trypomastigotes are curved, assuming the shape of the letters C, S, or U. - Unlike Leishmania, the kinetoplast of the trypomastigote is posteriorly located, with the single large nucleus located anterior to it. - The trypomastigotes are visible in the peripheral blood. Trypanosoma cruzi Epidemiology and Pathogenesis The parasite is found primarily in South and Central America Transmitted by the bite of the reduviid or triatomid bud (Triatoma or “cone nose” bug or “kissing bug”). Usually transferred to a human host when the feces of the bug containing the infective trypomastigotes is deposited near the bite site. The feces are then introduced into the bite site when the host scratches the bite area. Other routes of transmission include: - Blood transfusion - Sexual intercourse - Transplacental transmission - Through the mucous membranes when the bite site is near the eye or mouth. Humans and animals (domestic cats and dogs, and wild species such as armadillo, raccoon, and rat) serve as reservoir hosts. The trypomastigotes invade the surrounding cells and transform into amastigotes. The amastigotes then reproduce leading to destruction of host cells. Then transformed back into trypomastigotes, which invade the blood, penetrate other cells in the body, and transform back into amastigotes. Different cell types may be affected. However glial cells, reticuloendothelial cells, and especially myocardial cells are the most frequently affected. The disease is primarily seen in rural areas because the reduviid bug lives in the walls of rural huts and feeds at night. Acute infection is rarely seen in the United States. Chronic infection is now seen with increasing frequency among immigrants from Latin America. Disease: Chagas Disease (American Trypanosomiasis) The acute phase of the disease begins with a nodule (chagoma) near the bite site and unilateral swelling of the eyelid with conjunctivitis (Romana’s sign). The eyelid swelling may be due to the bug feces being accidentally rubbed into the eye. Accompanied by fever, chills, malaise, myalgia, and fatigue. Patients may recover or may enter the chronic phase. Characterize the chronic phase of Chagas disease: - Hepatosplenomegaly - Enlargement of lymph nodes (lymphadenopathy) - Myocarditis with cardiac arrhythmia. Cardiac muscle is the most frequently and most severely affected tissue. - Loss of tone of the colon and esophagus due to destruction of the Auerbach’s plexus may lead to abnormal dilatation of these organs, called megacolon and megaesophagus, respectively. CNS involvement may also be seen in the form of meningoencephalitis and cysts. Death may occur due to cardiac failure and arrhythmias. Other diagnostic methods that can be used include: - bone marrow aspiration, - muscle biopsy, - culture on special medium, and - xenodiagnosis. Xenodiagnosis entails allowing an uninfected laboratory raised reduviid bug to feed on an infected patient. After several weeks, the intestinal contents of the bug are examined for the presence of the parasite. Serologic tests can also be helpful. Both xenodiagnosis and serologic tests are useful in the chronic form of the disease. Treatment benznidazole and nifurtimox - The drugs of choice for treatment are but these are less effective during the chronic phase of the disease. Alternative agents are allopurinol and ketoconazole Prevention and Control 1. Prevention involves protection from the bite of the reduviid bug 2. Improvement of housing conditions, and insect control. 3. Education regarding the disease and its transmission is also helpful. Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense Laboratory Diagnosis Acute disease is diagnosed by the finding of trypomastigotes in thick or thin films of the patient’s blood. Epidemiology and Pathogenesis The two species are similar in morphology and life cycle. Their life cycles involve the tsetse fly (Glossina) as the vector. Humans are the reservoir for T. brucei gambiense, while domestic animals (especially cattle) and wild animals serve as the reservoir for T. brucei rhodesiense. The infective and pathogenic stage is the trypomastigote. The trypomastigotes spread from the skin to the blood then to the lymph nodes and the brain. A demyelinating encephalitis occurs leading to the characteristic manifestations of the disease. T. gambiense infection (West African or Gambian Sleeping Sickness) is chronic while T. rhodesiense infection (East African or Rhodesian Sleeping Sickness) is more rapidly fatal. The disease is endemic in sub– Saharan Africa which is the natural habitat of the tsetse fly. T. gambiense causes disease along the water courses in West Africa while T. rhodesiense causes disease mostly in the arid regions of East Africa. Disease: African Sleeping Sickness The initial lesion is an indurated ulcer called chancre at the site of the insect bite. Intermittent weekly fever and lymphadenopathy then develop. Enlargement of the posterior cervical lymph nodes (Winterbottom’ssign) is commonly seen. Other manifestations seen during this stage include: - red rash accompanied by pruritus - localized edema, and - a delayed pain sensation (Kerandel’s sign). The encephalitis is characterized by: - Headache insomnia, and mood changes. Muscle tremors slurred speech, and apathy follow, progressing to somnolence (sleeping sickness) and coma. Untreated disease is fatal. Trypanosoma brucei rhodesiense is more virulent than Trypanosoma brucei gambiense. Infection with the parasite has a shorter incubation period. Winterbottom’s sign may not be seen. There is no lymphadenopathy and CNS involvement occurs early in the course of the disease. A rapid and fulminating disease may follow with the parasite spreading in the blood. Death is seen usually within 9– 12 months following infection in untreated patients and may be due to glomerulonephritis and myocarditis. Laboratory Diagnosis Microscopic examination of Giemsa-stained slides of the blood, lymph node aspirations and CSF will reveal the trypomastigotes during the early stages of the disease. Aspiration of the chancre or enlarged lymph nodes may also reveal the parasites. Parasites are isolated from the CSF of patients with CNS involvement. Serologic tests can also be helpful as well as detection of the presence of IgM and proteins in the CSF of patients. The presence in the serum and/or CSF of IgM is considered diagnostic. - Treatment Several drugs are available for the treatment of both East African and West African Sleeping Sickness, which include melarsoprol, suramin, pentamidine, and eflornithine (Zeibig, 2013). The choice of drug will depend on whether the patient is pregnant or not, the age of the patient, and the stage of the disease. Prevention and Control Preventive measures involve protection against the bite of the fly. 1. Use of netting and protective clothing are recommended. 2. Use of fly traps 3. Insecticides may be helpful. 4. Clearing the forest around the villages are also helpful measures. Subphylum Apicomplexa:Plasmodium spp. Important Properties and Life Cycle Malaria is caused by five plasmodia species: - Plasmodium vivax, - Plasmodium malariae, - Plasmodium ovale, - Plasmodium knowlesi, and - Plasmodium falciparum. The vector and definitive host is the female Anopheles mosquito. The sexual cycle (sporogony) occurs primarily in mosquitoes, and The asexual cycle (schizogony) occurs in humans (intermediate hosts). The infective stage is the sporozoite from the saliva of the biting mosquito, which is taken up by the liver cells. This is called the exoerythrocytic phase. Multiplication and differentiation of sporozoites into merozoites occur during this stage. P. vivax and P. ovale produce a latent form (called hypnozoite or sleeping form) in the liver, which is the cause of the relapse or recrudescence seen in vivax and ovale malaria. Merozoites (pathogenic stage) are released from liver cells and infect the red blood cells. The parasite’s life cycle now enters the erythrocytic phase. These merozoites multiply and are eventually released to infect other red blood cells. The periodic release of merozoites causes the typical recurrent symptoms seen in malaria patients. Some merozoites then develop into microgametocytes (male gametocytes) and macrogametocytes (female gametocytes). The gametocyte containing red blood cells are ingested by the mosquito during feeding. Sexual reproduction then ensues. Epidemiology and Pathogenesis Infection with plasmodia occurs worldwide. It occurs primarily in tropical and subtropical areas, especially in Asia, Africa, and Central and South America. Sixty nine percent (69%) of cases in the Philippines are due to Plasmodium falciparum while the remaining 31% are due to Plasmodium vivax (World Malaria Report 2013). The primary vector is Anopheles flavirostris, which breeds in clear, slow flowing streams near foot hills and forests. In the 2014 Asia Pacific Malaria Elimination Network (APMEN) VI held in Makati City, Philippines. Secretary of Health Doctor Enrique Ona reported an 83% reduction in malaria cases from 2005 to 2013, with a 92% decrease in malarial deaths. Secretary Ona also reported that of 53 known provinces that are endemic for the disease, 27 have already been declared malaria free, which are: - Cavite - Batangas - Marinduque - Catanduanes - Alba - Masbate - Sorsogon - Camarines Sur - Iloilo - Aklan - Capiz - Guimaras - Bohol - Cebu - Siquijor - Western Samar - Eastern Samar - Northern Samar - Northern Leyte - Southern Leyte - Biliran - Camiguin - Surigao del Norte - Benguet - Romblon - Batanes - Dinagat Islands. The main mode of transmission of malaria is the bite of the female mosquito vector. The parasite can also be transmitted through blood transfusion (transfusion malaria), intravenous drug abuse with sharing of IV needles (“main line malaria”), and transplacental transmission (congenital malaria). Most of the pathologic findings result from the destruction of red blood cells. P. falciparum and P. knowlesi can infect both young and old red blood cells leading to high levels of parasitemia. P. vivax and P. ovale mainly infects young red blood cells, while P. malariae infects old red blood cells. Plasmodium knowlesi is a natural parasite of macaque monkeys throughout the Southeast Asia region. Cases of infection have been noted in Thailand, Singapore, Brunei, Indonesia, Myanmar, Vietnam, and the Philippines (Murray, 2014). The red blood cells infected by P. knowlesi have normal morphology. All developmental stages of the parasite may be seen in the peripheral blood. Disease: Malaria Paroxysms of malaria are divided into three stages: 1. cold stage 2. hot stage, and 3. the sweating stage. These paroxysms are considered partially as allergic responses to the schizonts and to the antigens released following the release of the merozoites. A malarial paroxysm presents with abrupt onset of chills (rigors) accompanied by headache, muscle pain (myalgia), and joint pains (arthralgia). This stage lasts for approximately 10–15 minutes or longer. Spiking fever lasting 2–6 hours follows, reaching up to 41 °C, accompanied by shaking chills, nausea, vomiting, and abdominal pain. This is then followed by drenching sweats. Patients usually feel well between febrile episodes. Splenomegalyis often present and anemia is prominent. The timing of the fever cycle is 72 hours for P. malariae, in which symptoms recur every 4th day (quartan malaria). Malaria caused by P. vivax, P. ovale, and P. falciparum recure every 3rd day (tertian malaria). P. falciparum causes malignant tertian malaria since it causes severe infection which is potentially life threatening due to extensive brain (cerebral malaria) and kidney damage. The dark color of the patient’s urine is due to kidney damage giving rise to the term “black water fever.” P. vivax and P. ovale cause benign tertian malaria that is characterized by relapses that can occur up to several years after the initial illness and is due to the latent hypnozoites in the liver. Most cases of P. knowlesi infection resembles infection in patients by other malarial parasites. A small number of cases of patients develops severe infection. The severity of the infection is due to the high parasitemia levels produced due to its ability to infect all stages of red blood cells and its 24 hour erythrocyte cycle (quotidian malaria). Laboratory Diagnosis The diagnosis of malaria is based on examination of Giemsa stained or Wright stained thick and thin smears of the blood. The thick blood smears are used for screening purposes The thin blood smears are used to differentiate the various Plasmodium species. The best time to take blood films is midway between paroxysms of chills and fevers or before the onset of fever. This is the time when the greatest number of intracellular organisms are present. Characteristic trophozoites will be seen within the infected red blood cells. P. falciparum will show characteristic crescent shaped or banana shaped gametocytes. Infection with P. falciparum is highly considered if there are > 10 infected red blood cells consisting only of ring forms. For P. malariae and P. knowlesi, demonstration of the characteristic rosette schizont is diagnostic. P. knowlesi should be suspected if there is a higher average merozoite count of 16/red blood cell as compared to 10–12/red blood cell of P. malariae. The presence of early trophozoite forms and two to three parasites per red blood cell (similar to P. falciparum) is more suggestive of P. knowlesi infection. Treatment chloroquine or parenteral quinine The drugs of choice for acute malaria infection. Chloroquine does not affect the hypnozoites of P. vivax and P. ovale. For vivax and ovale malaria, Primaquine is given to destroy the hypnozoites. For chloroquine resistant strains of P. falciparum Other agents may be used including mefloquine + artesunate, artemether lumafantrine, atovaquone proguanil, quinine, quinidine, pyrimethamine sulfadoxine (Fansidar), and doxycycline (Murray, 2014). Artemisin based combination therapies (ACTs) are now recommended for uncomplicated malaria and for chloroquine resistant vivax malaria. Artesunate is the drug of choice for severe malaria, in combination with either amodiaquine, mefloquine, or sulfadoxine pyrimethamine. P. knowlesi infection is managed similar to P. falciparum due to its potential to produce severe infection Prevention and Control Chemoprophylaxis of malaria for travelers to endemic areas consists of mefloquine or doxycycline. 1. Travelers to areas where the other plasmodia are found should take chloroquine starting two weeks before arrival and continued for 6 weeks after departure, followed by a 2-week course of primaquine if exposure was high. Other preventive measures include: 2. Avoidance of the bite of the vector through the use of mosquito netting 3. Window screens 4. Protective clothing, and 5. insect repellants. The mosquitoes usually bite from dusk to dawn, so protection is important during the night. Reduction of mosquito population is also helpful, including the use of insecticide sprays, as well as drainage of stagnant water in swamps and ditches. Phylum Apicomplexa:Toxoplasma gondii Important Properties and Life Cycle The definitive host of the parasite is the domestic cat or other felines Humans and other mammals serve as the intermediate hosts. The parasite develops in the intestinal cells of the cat and passes to the tissues through the bloodstream. These are then passed in the cat’s feces and mature into infective oocysts in the external environment. Infection in humans begins with the ingestion of oocysts (infective form) in undercooked meat or from contact with cat feces. In the small intestines, the oocysts rupture into trophozoites (tachyzoites or bradyzoites). Tachyzoites are the rapidly multiplying forms responsible for the initial infection Bradyzoites are shorter, slow growing forms seen in chronic infections. Epidemiology and Pathogenesis Infection by T. gondii occurs worldwide. Infection is usually sporadic but outbreaks associated with ingestion of raw meat or contaminated water can occur. Individuals who are severely immunocompromised are more likely to develop severe disease. The parasite can be transmitted in two ways: 1. ingestion of improperly cooked meat of animals that serve as intermediate hosts, and 2. ingestion of oocyst from contaminated water. Transplacental transmission may occur: 1. with severe consequences on the fetus. 2. Sharing of needles by IV drug abusers 3. blood transfusion are less common modes of transmission of the parasite. Disease: Toxoplasmosis 1. Infection in immunocompetent individuals - usually asymptomatic. - Acute infection may manifest non specific symptoms such as chills, fever, headache, and fatigue. This may be accompanied by inflammation of lymph nodes (lymphadenitis). - Chronic infection may manifest with lymphadenitis, hepatitis, myocarditis, and encephalomyelitis. Chorioretinitis leading to blindness may also occur 2. Congenital infection - occurs in infants born to mothers who were infected during pregnancy. - The manifestations vary depending on when the infection was acquired. - Infection during the first trimester of pregnancy may result to miscarriage, stillbirth, or severe infection (encephalitis, microcephaly, hydrocephalus,mental retardation, pneumonia). - If the infant acquires the infection during the last trimester, symptoms may not develop until months to years after delivery. - The most common manifestation is chorioretinitis with or without blindness. 3. Infection in immunocompromised hosts - usually manifest with neurologic symptoms similar to patients with diffuse encephalopathy, meningoencephalitis, or brain tumors. - Reactivation of latent toxoplasma infection is common. Other sites of infection include the lungs, eye, and testes. Laboratory Diagnosis Demonstration of high antibody titers through immunofluorescence assay is essential for the diagnosis of toxoplasma infection. Microscopic examination of Giemsa stained preparations will show the crescent shaped trophozoites during the acute infection. Cysts may be seen in the tissues. Prenatal diagnosis can be done through ultrasonography and amniocentesis with PCR analysis of the amniotic fluid (method of choice). Treatment Infection in immunocompetent hosts is usually self-limiting and does not require specific therapy. The regimen of choice for immunocompromised patients, especially those with AIDS, is initial high dose pyrimethamine plus sulfadiazine given for an indefinite period. Alternative regimen for those who develop symptoms of drug toxicity is clindamycin plus pyrimethamine. For pregnant women, clindamycin or spiramycin may be given. Prevention and Control 1. The most effective preventive measure is through adequate cooking of meat. 2. Pregnant women should refrain from eating undercooked meat 3. Should avoid contact with cats and refrain from handling litter boxes. Cats should not be fed raw meat. Microbiology and parasitology Final lecture Cestodes General Properties of Cestodes Cestodes are classified under the subkingdom Metazoa, phylum Platyhelminthes. These parasites are considered as primitive worms. They do not possess a digestive system nor a nervous system. They absorb nutrients and eliminate waste products through their outer surface called the tegument. Commonly known as tapeworms These parasites are flat and consist of three distinct regions—the head, neck, and body (proglottids). The head contains an organ of attachment called the scolex, which may consist of either hooks, suckers, or sucking grooves. In some species, the scolex has a fleshy extension called a rostellum to which hooks may be attached. The body is divided into multiple segments (hence, the name tapeworm) called proglottids. A series of proglottids is called strobila (plural strobili). All cestodes are hermaphroditic (self fertilizing) with each proglottid containing both male and female reproductive organs. Each proglottid, therefore, is capable of laying eggs (now called a pregnant proglottid or gravid segment). The neck serves as the region of growth and connects the head to the body of the worm. The worm grows by adding new proglottids from the neck. The oldest proglottids are found at the most distal part of the body of the parasite. A typical cestode life cycle is divided into three stages: - Egg - Larva - adult worm. For the majority of cestodes, the egg contains an embryo called the oncosphere, which represents the first larval or motile stage. It is equipped with small hooks (called hooklets) that eventually enable the parasite to pierce the wall of the intestines. The eggs are excreted in the feces of infected hosts and are transmitted to the intermediate hosts (cattle, pig, or fish). Infection in humans is usually acquired through ingestion of the undercooked or raw flesh of the intermediate host containing the infective larvae. After ingestion, the ingested larvae are transformed into adult worms in the intestines of the infected host. The adult worm then undergoes self-impregnation with the gravid segment rupturing to release the eggs in the intestines. These eggs are then passed out to the external environment during defecation. Intestinal Cestodes Taenia saginata (Beef Tapeworm) Important Properties and Life Cycle The intermediate host is cattle where the eggs enter the blood vessels within the cattle’s intestines. The eggs are then transported to the skeletal muscles of the cattle where they develop into cysticerci (larvae). Infection with the beef tapeworm is acquired by ingestion of improperly cooked or raw beef containing the infective larva (called cysticercus). These larvae then mature into adult worms (pathogenic stage) in the small intestines within a period of approximately three months. These tapeworms are known to achieve a length of as much as 10 meters. Humans serve as the definitive hosts. The eggs of Taenia saginata are usually indistinguishable from the eggs of the pork tapeworm Taenia solium. Both species may be differentiated by the appearance of their scolices and the structures of their proglottids. The scolex of Taenia solium contains a rostellum while that of Taenia saginata does not. Taenia saginata proglottid is rectangular and contains more uterine branches (about 15–30) in comparison with Taenia solium which is square in appearance containing about 7–15 uterine branches. Epidemiology and Pathogenesis Taenia saginata infection is common in areas of the world where beef is routinely eaten, especially undercooked beef. It has been found to be endemic in Eastern Europe, Russia, Eastern Africa, and Latin America (Centers for Disease Control and Prevention). The adult worms do not produce significant damage in the small intestines. Disease: Taeniasis Majority of patients are asymptomatic. Those with high worm burden may complain of diarrhea, abdominal pain, loss of appetite with resultant weight loss, and body malaise. The gravid proglottids may reach the anus where egg laying may occur resulting in itchiness in the anal region (pruritus ani). Laboratory Diagnosis Examination of fecal specimen from infected patients is the procedure of choice. Eggs or gravid proglottids may be recovered from the stool although eggs are less often found than the proglottids Treatment Praziquantel- The drug of choice against the adult worm Prevention and Control 1. Proper waste disposal and sanitation practices as well as the adequate cooking of beef are the main preventive measures for taeniasis. 2. Freezing of beef meat for approximately 10 days may kill the encysted larvae. Prompt treatment of infected persons help prevent spread of the disease. Taenia solium (Pork Tapeworm) Important Properties and Life Cycle Infection with the pork tapeworm is acquired through ingestion of improperly cooked or raw pork meat which contains the infective larva called cysticercus cellulosae. Unlike the beef tapeworm, Taenia solium infection can also occur following the ingestion of food or water contaminated with human feces that contain the eggs of the parasite. Therefore, unlike the beef tapeworm, Taenia solium has two infective stages - eggs - larvae Autoinfection may also occur. Pigs serve as the intermediate host while humans serve as both intermediate and definitive hosts. There are two scenarios that can occur depending on which infective stage entered the human host. In cases where infection is acquired through ingestion of undercooked or raw pork meat, the infective stage is the larval form which transforms into adult worm in the intestines of infected individuals. In this instance, humans serve as the definitive hosts. On the other hand, ingested worm eggs hatch in the small intestines, burrow through the wall of the intestines into a blood vessel, and disseminate to various organs. In this instance, humans serve as intermediate hosts. Epidemiology and Pathogenesis T. solium infection is more prevalent in underdeveloped communities with poor sanitation and where people eat raw or undercooked pork. Higher rates of illness have been seen in people in Latin America, Eastern Europe, sub–Saharan Africa, India, and Asia (Centers for Disease Control and Prevention). Adult worms produce little damage in the intestines. Encysted larvae may produce damage in the tissues where they disseminate. For instance, in the brain, they may manifest as space occupying lesions. Although the larvae may encyst in various tissues of the body, they evoke little inflammatory response. When the encysted larvae die, they may release substances that may induce an allergic reaction in the host which may potentially be fatal due to the development of anaphylactic shock. Disease 1. Taeniasis - the disease produced by the adult worm. - Most cases are asymptomatic but in the presence of high worm burden, manifestations may be similar to beef tapeworm infection. 2. Cysticercosis - the result of larval encystation in various tissues of the body. The most common involvement is that of the skeletal muscles where patients may complain of muscle pain. - Cyticercosis of the brain (neurocysticercosis) is the most feared and most severe involvement. - It may present with symptoms associated with increased intracranial pressure such as seizures, headache, and vomiting. - Ocular cysticercosis may lead to visual disturbances due to development of inflammation of the uvea (uveitis) and retina (retinitis). Laboratory Diagnosis Microscopic examination of stool specimen from infected persons is the diagnostic procedure of choice in patients with taeniasis. Demonstration of ova or proglottids may help establish the diagnosis. The demonstration of the typical morphology of the scolex can differentiate pork tapeworm from beef tapeworm. For cysticercosis, diagnostic procedure depends on demonstration of the cyst in tissue, through biopsy or CT scan. Treatment Praziquantel - The drug of choice for treatment of intestinal infection For cysticercosis, praziquantel may also be effective but it is usually not recommended for ocular and CNS involvement. Alternative drugs include albendazole, paromomycin, and quinacrine hydrochloride. Surgical removal of the larvae may be necessary. Cases of neurocysticercosis- Anti convulsants may be given, Prevention and Control Important preventive measures for pork tapeworm infection are the same as that for beef tapeworm and include: 1. proper waste disposal 2. sanitary measures thorough cooking of pork meat, and the prompt treatment of infected persons to prevent the spread of the parasite. Diphyllobothrium latum (Broad Fish Tapeworm) Important Properties and Life Cycle The longest of the tapeworms, the fish tapeworm can reach a length of about 13 meters. Its eggs consist of ciliated larvae called coracidia (s. coracidium). One end of the egg is occupied by a lid structure called an operculum. Its scolex contains a pair of long sucking grooves. The gravid segments contain a uterine structure that is centrally located and assumes a rosette formation. Human infection with D. latum is through ingestion of improperly cooked or raw fish containing the plerocercoid (infective stage), the precursor larval stage. After ingestion, the plerocercoid attaches to the intestinal mucosa and matures into the adult worm. The adult worm self-fertilizes and the eggs are passed out with the stool. If the eggs come to contact with fresh water, the coracidium hatches and is ingested by the first intermediate host, a tiny crustacean called a copepod (Cyclops sp.). After ingestion, the coracidium develops into the larval stage called the procercoid. The copepod is then eaten by a freshwater fish (second intermediate host) where the procercoid develops into the plerocercoid. Definitive hosts for the parasites are humans and other fish eating mammals such as dogs, cats, bears, and seals. Epidemiology and Pathogenesis D. latum infection occurs in countries where raw freshwater fish is consumed. Little damage is produced in the small intestines of the human hosts. In some individuals, the parasite may compete with the host for vitamin B12, leading to a deficiency of this vitamin. Disease: Diphyllobothriasis 1. Asymptomatic disease – the most common presentation among most individuals infected with the parasite. 2. Diphyllobothriasis– may manifest with symptoms of gastrointestinal involvement, which may include diarrhea and abdominal discomfort. When the adult worm attaches itself to the jejunum and ileum, the patient may develop deficiency of vitamin B12, leading to anemia similar to pernicious anemia and is characterized as megaloblastic anemia resulting from lack of maturation of red blood cells. Laboratory Diagnosis Diagnosis is based on finding of the characteristic eggs and/or the proglottids (less frequent) in a stool specimen. Treatment The drug of choice for the treatment of diphyllobothriasis is praziquantel. An alternative drug is niclosamide. Prevention and Control Preventive measures include: 1. Proper sanitary procedures, thorough cooking of fish prior to consumption, and the prompt treatment of infected individuals to 2. Prevent spread of the parasite. Freezing of the fish for 24–48 hours at –18 °C can kill all larvae. Hymenolepis nana (Dwarf Tapeworm) Important Properties and Life Cycle H. nana is different from the other tapeworms because it does not require an obligatory intermediate animal host. The eggs are directly infectious and humans get the infection after the accidental ingestion of the eggs of the parasite. This may occur after ingestion of fecally contaminated food or water. One may also acquire the eggs by touching one’s mouth with contaminated fingers or through ingestion of contaminated soil. Accidental ingestion of rice or flour beetles containing the infective larvae and that may have gotten into food is another way by which the infection may be acquired. Rodents serve as additional source of infection. Once the eggs (infective stage) gain entrance into the human host after ingestion of contaminated food and water, the eggs transform into cysticercoid larvae. The larvae mature into adult worms capable of self-reproduction. Eggs are released after disintegration of the gravid segments. There are two pathways for the eggs - the eggs may be passed to the outside environment through the feces or some of the eggs may remain inside the human host. - Those that remain inside the human host hatch into larvae and mature into adult worms, thereby starting a new cycle within the human host. This type of re infection is called autoinfection. Epidemiology and Pathogenesis The dwarf tapeworm is the most common tapeworm recovered in the United States. It has a worldwide distribution and is also found in East Asia and the Philippines. It is common in areas with inadequate sanitation and hygiene. Children and persons living in crowded areas are at risk of developing infection. The parasite produces little damage in the small intestines Disease: Hymenolepiasis Most patients are asymptomatic. In cases of high worm burden, patients may complain of : - Nausea - Weakness - loss of appetite - diarrhea, and - abdominal pain. - In young children with heavy infection, anal itchiness (pruritus ani) may occur leading to headaches due to difficulty sleeping. It can be confused with a pinworm infection. Autoinfection may lead to hyper infection syndrome which can result in secondary bacterial infection and spread of the worms to other tissues of the body. Laboratory Diagnosis Diagnosis is established by finding of the characteristic eggs in stool specimen. Treatment Praziquantel is the drug of choice. Niclosamide can be an alternative drug. Prevention and Control Important preventive measures include: 1. Proper hygiene 2. Waste disposal 3. control of transport host population 4. Rodent control 5. Proper storage of grains 6. Flour must be observed to prevent infestation with flour and grain beetles. 7. Prompt treatment of infected individuals must be instituted to prevent the spread of the parasite. and water contaminated by dog feces or through contact with contaminated dog feces. Eggs transform into larvae in the intestines, penetrate the intestines, and migrate through the bloodstream to different tissues in the body, particularly the liver and the lungs. The hydatid cyst (pathogenic stage) then develops in the infected tissues. Dogs acquire the parasite by eating the visceral organs of the intermediate host. Extra Intestinal Cestode Echinococcus granulosus (Dog Tapeworm or Hydatid Tapeworm) Important Properties and Life Cycle Infection with E. granulosus is primarily a zoonotic type of infection. Dogs are the most important definitive hosts Sheep are usually the intermediate hosts. Humans are considered as accidental and dead end hosts. The eggs of E. granulosus are identical to those of Taenia spp. and are thus not diagnostic. The diagnostic stage of the parasite is its larval form, which is encased in a cyst wall and is called the hydatid cyst. Infection is acquired after ingestion of eggs (infective stage) from food Epidemiology and Pathogenesis E. granulosus infection is common in Africa, Europe, Asia, the Middle East, Central and South America, and in rare cases, North America (Center for Disease Control and Prevention). The embryos develop into large, fluid filled hydatid cysts, which act as space occupying lesions. In addition, the cyst fluid contains antigens that can sensitize the host. Rupture of the cyst, either spontaneously or during trauma or surgical removal, may lead to the release of these antigens leading to anaphylaxis and widespread dissemination of the parasite. Disease: Echinococcosis, Hydatid Cyst Disease, Hydatid Disease, Hydatidosis Most patients are asymptomatic during the early stages of the disease. However, as the cysts enlarge, necrosis of the infected tissues occur. Involvement of the liver may result in obstructive jaundice. Patients with lung involvement may manifest with cough, chest pain, and shortness of breath. Other organs that may be infected include the spleen, kidneys, heart, bone, and central nervous system, including the brain and eyes (Center for Disease Control and Prevention). Cyst rupture may lead to anaphylactic shock leading to death of the patient. Laboratory Diagnosis There are several ways by which E. granulosus infection can be diagnosed. These include: 1. examination of biopsy specimen; 2. serologic tests (e.g., ELISA or indirect hemagglutination test); and 3. radiography to demonstrate the hydatid cysts (e.g., CT scan or ultrasound). Care should be exercised when doing biopsy to prevent rupture of the cyst. Treatment In cases when surgery is possible, removal of the cyst has been considered as the treatment of choice. However, medical management alone may prove effective, especially if the cyst is located in inaccessible areas. Drugs that have been proven effective include mebendazole, albendazole, and praziquantel. Prevention and Control 1. Improvement of personal hygiene practices 2. prevention of contamination of food and water with dog feces 3. avoidance of feeding pet dogs with contaminated viscera 4. the prompt treatment of infected canines and humans are some measures to prevent the spread of the parasite. 5. Chemoprophylaxis should be given to dogs in endemic areas. 6. Health education is essential.