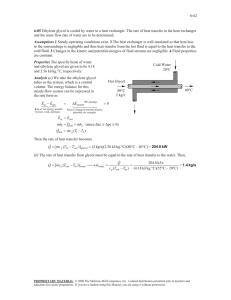
УДК 621.59 COMPARATIVE ANALYSIS OF NATURAL GAS DRYING METHODS Saftly А.1 (postgraduate) Supervisor - Candidate of Technical Sciences, Associate Professor Sokolova.E.V1 ITMO university e-mail: adhamsaftly1994@gmail.com Abstract Natural gas processing plays a key role in the natural gas supply chain. Dehydration is one of these processes where the presence of water can cause mechanical problems during pipeline transport or liquefaction process. This article discusses the common processes used for water removal and gives a comparison between two of the most used methods in dehydration natural gas (the required level of water concentration in the resulting gas, the regeneration process, the needed quantity of adsorbent/glycol, and finally the main factors that affect the adsorption and absorption process) Keywords Dehydration, adsorption, absorption, molecular sieve, glycol, water concentration, process regeneration Natural gas is the most energy-efficient fossil fuel. The flame temperature of natural gas is higher than that of other fuels because it contains more hydrogen relative to carbon. The autoignition temperature of natural gas depends on its composition and is in the range of 540–600 °C, which is much higher than that of diesel fuel or gasoline. Another advantage of using natural gas is the impact on global warming, where it emits a very low level of emissions in comparison with coal. However, one of the problems that are faced in Natural gas is the gas impurities such as water [1], where gas usually contains water in liquid and/or vapor form at the well (the source). Another reason for the presence of water is as a result of chemical reaction with an aqueous solution to remove acid gases CO2 and H2S, so the resulting gas is saturated with water [2,3]. If the water level is more than 0.1ppmv for the cryogenic process or more than 10 ppmv for gas in pipes, this may cause: - forming solid hydrates that can plug valve fittings or even pipelines; - water condensation in pipes, which may cause erosion and corrosion; - decrease in the heating value of the gas; - in a liquefaction plant, hydrate formation and freezing in the cryogenic section of the plant. 1. Dehydration Process – There are many applications that lead to the drying process, depending on the circumstances. Absorption, Adsorption, membrane, and refrigeration process are the most known examples. In general, Absorption and Adsorption are the most used in natural gas dehydration, and that is why they are compared. 1.1 Adsorption – This process helps to reach the lowest level of water between a lot of applications (depending on the used adsorbent). 0.1 ppmv range can be reached by Physical adsorption. The commonly available adsorbents and their uses are described as follows: - silica gel; a high concentration of water in feed gas (>1mol%), a low level of water in the resulting gas (not enough for the cryogenic process); - activated alumina, al2o3; moderate levels of water in the feed gas, does not require a low level of water in the resulting gas (not enough for the cryogenic process); - molecular sieves; the only adsorbent that can achieve the cryogenic processing requirements for dehydration; The 4A molecular sieve is the most used, and 3A is the second option, depending on the circumstances [1]. 1.2 Absorption by glycol – Glycols are the most widely used absorption liquids as they approximate the properties that meet the commercial application criteria. Several glycols are suitable for industrial applications like ethylene glycol C2H6O2 (EG), diethylene glycol C4H10O3 (DEG), triethylene glycol C6H14O4 (TEG) and propylene glycol C3H8O2. TEG is the most frequently used because it offers the best compromise between solvent losses and initial cost while DEG is used in cold climates because of its lower viscosity at lower contactor temperatures and EG is used when frequent brine carryover into the contactor occurs because it can hold more salt than the other glycols [2]. The normal process with glycol TEG can reach the 10 ppmv range of water level in the resulting gas in a physical absorption process, however, with some enhancement, it may obtain the needed requirements for cryogenic processing. 2. Cycles and regeneration process for Molecular sieves: 2.1 Adsorption by Molecular sieves cycle and regeneration – the regeneration step requires a high temperature, so this step is considered an energy-intensive (Recommended regeneration temperature for Molecular sieves 4A is between 200–315 °C) (Schematic1). Schematic 1 a two-bed adsorption unit. Valving shown is with adsorber 1 in drying cycle and adsorber 2 in regeneration cycle [2] The regeneration step in Molecular sieves is a little complicated, so it includes heating the bed walls, the adsorbent, the heat to remove water from the adsorbent, and the losses are taken into consideration. The next equations show the heat duty needed in the regeneration cycle 1. Desorption step (removing water from the adsorbent) Q1=4200 kJ/kg for every kg of water on the bed. (1) 2.Molecular sieves Q2=1.0 kJ/kg for every kg of sieve on the bed* (trg - t1). (2) 3.the vessel walls Q3=0.5 kJ/kg for every kg of steel in the walls *(trg - t1). (3) 4.the losses to surroundings assuming external insulation Q4=0.1(Q1+Q2+Q3). (4) Where trg is the regeneration temperature of the gas that enters the bed minus (28°C), and t1 is the gas temperature during adsorption. The total estimated heat of regeneration is then Q tot=2.5(Q1+Q2+Q3+Q4)=2.75(Q1+Q2+Q3). (5) Where the factor of 2.5 corrects for the change in temperature difference (in–out) across the bed with time during the regeneration cycle. 2.2 Absorption by Glycol TEG cycle and regeneration the regeneration step here requires a lower temperature than adsorption (the maximum Recommended regeneration temperature for glycol TEG is 196 - 204 °C) (Schematic 2). Schematic 2 typical glycol dehydrator unit [2] The reboiler supplies the needed heat to the regeneration step. The reboiler total energy that is utilized can be calculated using the following equation Q =900+966(G). (6) Where Q is regenerator heat, Btu/lb H2O removed; and G is the glycol-to-water ratio, gal TEG/lb H2O removed. Where lb=0.45359237 kg, gallon=3.78541 liter, btu=1.05506 joule 3. the amount of adsorbent/glycol in a cycle: Here two points are discussed • how much adsorbent/glycol is needed to absorb 1 kg of water; • calculation of the amount of adsorbent/glycol in any cycle. 3.1 Molecular sieves – The Engineering Data Book (2016b) states that the design water content of a 4A molecular sieve, when at equilibrium with saturated gas at (24°C), will be 13 Kg H2O/100 Kg sieve. And for a new molecular sieve holds about 20 Kg H2O/100 Kg sieve The required amount of Adsorbent G (kg) is determined by the formula: 𝑄.∆W.t 𝐺= (7) α where Q is the amount of dried gas, m3/h; ∆W is the amount of moisture absorbed by the adsorbent, kg/m3; α – the dynamic activity of the adsorbent; t is the time of adsorbent operation h. 3.2 Glycol TEG – The glycol circulation rate is usually between 17 liters and 50 liters of TEG per Kg of water. For absorbers with more than three equilibrium stages (a typical absorber design), 25 liters TEG/kg water is sufficient[4]. Absorber operating experience has shown that it should circulate at least 25 liters per 1 kg of absorbed water. The amount of concentrated absorbent solution G (kg/h) required for drying the gas is determined by the formula: 𝑄.(𝑊𝐻 −𝑊𝐾 ).X 𝐺= (8) X1−X2 where Q is the amount of dried gas, thousand m3/h; WН, Wk – moisture content of incoming and dried gases, respectively, kg/thousand m3; X1, X2 – absorbent concentration in fresh and saturated solutions, wt %. 4. Factors that affect the dehydration process and losses 4.1 Molecular sieves - a limited change in the bed Pressure (not more than 6 kPa/s) helps to avoid adsorbent attrition, so monitoring the pressure drop provides us with information about the bed health -the presence of oxygen, even in a trace amount, affect bed life and performance in a variety of ways (reactions happen and lead to an increase of the bed temperature in the regeneration step and reduce the capacity of the adsorbent), lowering the regeneration temperature to (150°C– 160°C) can help in avoiding these reactions: -the more regeneration cycles, the more deterioration in the molecular sieve; -if H2S is present, then an oxidation to elemental sulfur happens. losses: common rates are about 35% loss of the capacity in a period of time between 3 to 5 year or about 4,000 cycles, Usually, a rapid loss occurs in the beginning and a gradual loss thereafter [2]. 4.2 Glycol TEG -For gas streams with a high CO2 concentration, equilibrium glycol losses can be much higher and should be considered when selecting the glycol [2]. -Oxygen reacts with the glycols to form corrosive acidic compounds. These compounds also increase the potential for foaming and glycol carryover. also, aldehydes can be formed and emitted from the still, these emissions are undesirable both from the standpoint of toxicity and odor [2]. -low ph accelerates glycol decomposition; -Water-soluble components increase the regenerator duty, and hydrocarbon impurities can cause foaming in the system. -an increase in circulation rate may decrease the reboiler temperature, lowering lean glycol concentration, and actually decrease the amount of water that is removed by the glycol; -long-term exposure of glycols to temperatures above 50°C results in glycol degradation. Losses it is estimated to be about 14 L/106 Nm3 from carryover if a standard mist eliminator is used. Other losses range between 6 L/106 Nm3 for high-pressure, low-temperature dehydration and 40 L/106 Nm3 for low-pressure, high-temperature dehydration. Losses from vaporization are negligible. Higher losses can result from foaming problems in either the contactor or regenerator or from glycol solution degradation due to high reboiler temperatures. Conclusion Molecular sieve is the only method that proved a high efficiency of dehydration that is needed for the cryogenic process, in addition, it can also help in removing mercaptan and co2(Dehydration on molecular sieves can replace amine treatment. If carbon dioxide content is less than 1%) A normal absorption by glycol cannot reach the needed level of water concentration for the cryogenic process, but an Enhanced TEG process developed by Skiff et al. (2003) claimed that less than 0.1 ppmv water was obtained by use of the Drizo™ process with a modified regeneration system and it consumes 30% energy less than the molecular sieve High water concentrations in the feed gas make the regeneration duty more expensive because about 50 % of the required energy in the adsorption cycle is related to the amount of water adsorbed. The technological schemes of installations using the absorption method are more complex compared to adsorption systems, but their operating and reduced costs are approximately three times lower however, the sizes of adsorbers are 2-3 times smaller than absorbers. References: 1. Mokhatab.S , Poe W.A , , Handbook of natural gas transmission and processing second edition ,The Boulevard, Langford Lane, Oxford, Kidlington, UK 2012, 802 pages. 2. Kidnay A.J , Parrish.W.R., and McCartney.D.G, Fundamentals of Natural Gas Processing Third Edition, Taylor & Francis Group, LLC, 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, 2020, - 472 pages 3. Baranov A.Y, Sokolova E.V, Ivanov L.V, Ikonnikova A.Y, Prospects for the development of LNG technologies in the Russian Federation, / Bulletin of the International Academy of Refrigeration.. 2023. № 1. С. 23-34. DOI: 10.17586/1606-4313-2023-22-1-23-34» 4. A. F. Beznosikov, M. I. Zaboeva, I. A. Sintsov, D. A. Ostapchuk, Development and operation of gas and gas condensate fields, Tyumen TIU 80s, 2016