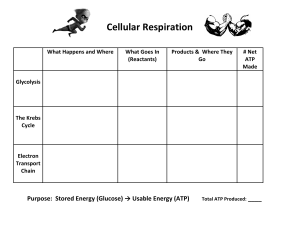
GLYCOLYSIS The main aim of digestion of carbohydrates is to release their building block units, which are catabolized, and the energy stored in the form of ATP molecules released for the performance of work in tissues. Every tissue has its requirement for glucose. In some cases like in the brain, the requirement is substantial while in others like erythrocytes, it is total. Glycolysis is derived from the Greek glykys, “sweet” or “sugar,” and lysis, “splitting”. In the process, a molecule of glucose is degraded in a series of enzyme catalyzed reactions to yield two molecules of the three-carbon compound pyruvate. Glycolysis may be considered as the pathway by which glucose is converted via fructose-1, 6-bisphosphate to pyruvate with a net generation of 2 molecules of ATP per molecule of glucose. This sequence of ten enzymatic reactions, which occur in the cytosol is probably the most completely understood biochemical pathway. The pathway plays a key role in energy metabolism by providing a significant portion of the energy utilized by most organisms. The glycolytic pathway is divided into two phases (preparative phase and pay off phase). In the preparative phase, glucose is phosphorylated and converted to glyceraldehydes-3-phosphate. The payoff phase is concerned with the oxidative conversion of glyceraldehydes-3-phosphate to pyruvate and the coupled formation of ATP and NADH. Page 1 of 8 Page 2 of 8 REACTIONS OF GLYCOLYSIS Hexokinase This is the first reaction of glycolysis (priming reaction) which involves the transfer of phosphoryl group from ATP to glucose to form glucose-6-phosphate (G-6-P) in a reaction catalyzed by hexokinase. A kinase is an enzyme that transfers phosphoryl groups between ATP and a metabolite. The different body tissues possess different isoenzymes of hexokinase. The metabolite that serves as the phosphoryl group acceptor for a specific kinase is identified by the prefix of the kinase name. Hexokinase is non-specific as it is contained in all cells that catalyze the phosphorylation of hexoses (D-Glucose, D-Mannose, D-Fructose etc.). Phosphorylation of glucose has its cellular advantage as it keeps the glucose in the cell and also keeps the glucose cellular concentration low to allow diffusion. At low glucose concentration prevalent in most cells phosphorylation is achieved by the activities of hexokinase. While at high glucose concentration in the liver phophorylation of glucose is achieved by the activities of insulin inducible glucokinase. Phosphoglucose Isomerase The second reaction of the glycolytic pathway is the conversion of G-6-P to Fructose-6phosphate (F-6-P) phosphoglucose isomerase (glucose-6-phosphate isomerase). This is the isomerisation of aldose to ketose. The reaction involves opening of ring, isomerisation and ring closure. Page 3 of 8 Phosphofructokinase This is the second ATP utilization step of glycolysis. The enzyme phosphofructokinase1(PFK-1) phosphorylates F-6-P to yield Fructose-1, 6-bisphosphate (F-B-P). PFK plays a central role in glycolysis as it catalyzes one the pathway’s rate limiting steps. Aldolase Aldolase catalyzes the fourth reaction of glycolysis resulting in the cleavage of F-B-P to two triose sugars glyceraldehydes-3-phosphate (GAP) and Dihydroxyacetone phosphtate (DHAP) in an aldo cleavage reaction. Triose Phosphate Isomerase One of the products of aldo cleavage reaction GAP continues along the glycolytic pathway. However, the DHAP and GAP are ketose-aldose isomers just as F-6-P and G-6-P. The interconversion occurs by the actions of triose phosphate isomerase via an intermediate and marks the end of the preparative phase of glycolysis. Page 4 of 8 Glyceraldehydes-3-Phosphate Dehydrogenase (GAPDH) This if the beginning of the payoff phase of the pathway resulting in the generation of high energy intermediates. In the presence of NAD+ and Pi, GAPDH catalyzes the oxidation and phosphorylation of GAP to 1, 3-Bisphosphoglycerate (1, 3-BPG) with concomitant generation of NADH. In this reaction the exergonic oxidative reactions drives the synthesis of the acyl phosphate 1, 3 BPG. Phosphoglycerate Kinase (PGK) This is the step for the first synthesis of ATP (substrate level phosphorylation) in the glycolytic pathway. In this reaction the phosphate moiety attached to position one of 1, 3 BPG is transferred to ADP to generate ATP and 3-phosphoglycerate (3-PG) by the activities of PGK. Phosphoglycerate Mutase (PGM) Page 5 of 8 In the eight reaction of glycolysis, 3-PG is converted to 2-phosphoglycerate (2-PG) by PGM. The mutase enzyme catalyzes the transfer of functional groups from one part of a molecule to another. The phosphate group at carbon 3 of 3-PG is transferred to carbon as seen in 2-PG. Enolase In the reaction catalyzed by enolase, 2-PG is dehydrated to generate phosphoenol pyruvate (PEP). The product of enolase activity PEP is the second high energy intermediate of the glycolytic pathway. Pyruvate Kinase (PK) This is the last reaction of the glycolytic pathway and the second point of ATP generation (substrate level phosphorylation). The PK couples the free energy of hydrolysis of PEP to synthesis of ATP and formation of pyruvate, the product of glycolysis. Page 6 of 8 REGULATION OF THE PATHWAY Since a metabolic pathway is a series of enzyme-catalyzed reaction, there exist flux control mechanisms that regulate the pathway. Most glycolytic reactions are reversible but three of them are markedly exergonic and considered physiologically irreversible. These reactions are catalyzed by hexokinase, PFK and PK and constitute the major site for regulation of glycolysis. The rate of glycolysis must be regulated to meet the needs of the intracellular and extracellular requirements. The degradation of glucose to pyruvate is regulated to meet two major cellular needs which include; ATP generation and the provision of building blocks for synthetic reactions, such as the formation of fatty acids. Most glycolytic reactions are reversible but three of them are markedly exergonic and considered physiologically irreversible. These reactions are catalyzed by hexokinase, PFK and PK and constitute the major site for regulation of glycolysis. Each of the irreversible reactions serves as a control site. Their activities are regulated by the reversible binding of allosteric effectors or by covalent modification. In addition, the amounts of these important enzymes are varied by the regulation of transcription to meet changing metabolic needs. Phosphofructokinase is the most important control element in the mammalian glycolytic pathway. High levels of ATP allosterically inhibit the enzyme in the liver (a 340-kd tetramer), thus lowering its affinity for fructose 6-phosphate. The presence of AMP reverses the inhibitory action of ATP, and so the activity of the enzyme increases when the ATP/AMP ratio is lowered. The inhibition of phosphofructokinase by H+ prevents excessive formation of lactic acid and a precipitous drop in blood pH. Glycolysis is a source of carbon skeletons for other biosynthetic pathways. Phosphofructokinase is inhibited by citrate, an intermediate in the citric acid cycle. Citrate inhibits phosphofructokinase by enhancing the inhibitory effect of ATP. In 1980, fructose 2,6-bisphosphate (F-2,6-BP) was identified as a potent activator of phosphofructokinase. Fructose 2,6-bisphosphate activates phosphofructokinase by increasing its affinity for fructose 6-phosphate and diminishing the inhibitory effect of ATP. Two enzymes regulate the concentration of fructose 2,6-bisphosphate regulator of glycolysis by phosphorylating fructose 6-phosphate and dephosphorylating fructose 2,6-bisphosphate. Fructose 2,6-bisphosphate is formed in a reaction catalyzed by phosphofructokinase 2 (PFK2), Page 7 of 8 a different enzyme from phosphofructokinase. Fructose 2,6-bisphosphate is hydrolyzed to fructose 6-phosphate by a specific phosphatase, fructose bisphosphatase 2 (FBPase2). Page 8 of 8