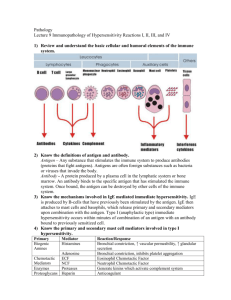
HYPERSENSITIVITY & AUTOIMMUNITY Angol Denish Calmax OUTLINE Overview Type I hypersensitivity Type II hypersensitivity Type III hypersensitivity Type IV hypersensitivity Autoimmunity References OVERVIEW TYPE I HYPERSENSITIVITY (Immediate Hypersensitivity)....1 HYPERSENSITIVITY The term hypersensitivity is used to describe immune responses which are damaging rather than helpful to the host. HISTORY Nearly 45 years ago Gell and Coombs proposed a classification scheme which defined 4 types of hypersensitivity reactions. TYPES OF HYPERSENSITIVITY The four types of hypersensitivity are: 1. Type I Hypersensitivity- IgE mediated 2. Type II Hypersensitivity- Antibody mediated 3. Type III Hypersensitivity- immune complex 4. Type IV Hypersensitivity- cell mediated The first three are mediated by antibody, the fourth by T cells. TYPE I HYPERSENSITIVITY (Immediate Hypersensitivity)....2 Testing for Immediate Hypersensitivity...1 In Vivo Skin Tests (Cutaneous or intradermal) -Cutaneous testing or a prick test: a small drop of material is injected into the skin at a single point. After 15 minutes, a WHEAL (raised & reddened part of skin) 3 mm greater in diameter than the negative control (saline) is POSITIVE - Intradermal test: A 1 mL tuberculin syringe is used to administer 0.01 to 0.05 mL of test solution between layers of the skin. After 15-20 mins, a WHEAL 3 mm greater in diameter than the negative control (saline) is POSITIVE In Vitro Tests: RIST- radioimmunosorbent test - Total IgE -competitive & non-competitive RAST- radioallergosorbent test - Antigen-Specific IgE Testing -non-competitive Microarray Testing Testing for Immediate Hypersensitivity...2 TYPE II HYPERSENSITIVITY (cytotoxic hypersensitivity) The reactants (antibodies) responsible for type II Hypersensitivity are IgG and IgM Triggered by antigens found on cell surfaces (altered selfantigens or heteroantigens) Promoting phagocytosis by both opsonization and activation of the complement cascade Examples: Transfusion Reactions, Hemolytic disease of the newborn (HDN), Autoimmune Hemolytic Anemia (Warm autoimmune hemolytic anemia or Cold autoagglutinins ) and Type II Reactions Involving Tissue Antigens(e.g. Goodpasture’s syndrome) Testing for Type II Hypersensitivity Coombs’ test Direct Coombs’ test (Direct antiglobulin testing, DAT) detects: - transfusion reactions -haemolytic disease of the newborn (HDN) -autoimmune haemolytic anaemia The indirect Coombs’ test is used in the cross-matching of blood to prevent a transfusion reaction. TYPE III HYPERSENSITIVITY Involves soluble antigen but also IgG or IgM and complement mediation. The soluble antigen combines with antibody, complexes are formed that precipitate out of the serum & deposit in the tissues if the immune system is overwhelmed Examples: Arthus Reaction, Serum Sickness Testing for Type III Hypersensitivity Detection of antibody by: -agglutination reactions using antigen-coated carrier particles e.g. red blood cells or latex particles -enzyme immunoassays Fluorescent staining of tissue sections to determine deposition of immune complexes in the tissues More general method of determining immune complex diseases is by measuring complement levels (decreased level shows antigen–antibody combination ) TYPE IV HYPERSENSITIVITY (Delayed hypersensitivity) Langerhans cells in the skin and macrophages in the tissue capture and present antigen to Th 1 cells. The Th1 cells are activated and release cytokines (e.g. IL-3, IFN-γ, TNF-β and TNF-α) that recruit macrophages and neutrophils, produce edema, promote fibrin deposition, and generally enhance an inflammatory response Cytotoxic T cells are also recruited, and they bind with antigen-coated target cells to cause tissue destruction Initial sensitization phase is1 to 2 weeks after the first contact with antigen Upon subsequent exposure to the antigen, symptoms typically take several hours to develop and reach a peak 48 to 72 hours NB: The rxn can only be transferred to other animals through transfer of T lymphocytes. Examples: Contact Dermatitis, Hypersensitivity Pneumonitis, Tuberculin-Type Hypersensitivity Testing for Delayed Hypersensitivity...1 The patch test (gold standard) -Be done when the patient is free of symptoms or when he or she at least has a clear test site. -A non-absorbent adhesive patch containing the suspected allergen is applied on the patient’s back, and the skin is checked for a reaction over the next 48 hours. -Redness with papules or tiny blisters is considered a POSITIVE test. -Final evaluation is conducted at 96 to 120 hours. Testing for Delayed Hypersensitivity (The patch test-gold standard)....2 ILLUSTRATION STRONG POSITIVE RESULT Testing for Delayed Hypersensitivity...3 Mantoux method skin test -Typically, 0.1 mL of the antigen is injected intradermally, using a syringe and a fine needle -The test site is read at 48 and 72 hours for the presence of induration (hardening). -An induration of 5 mm or more is considered a POSITIVE test. -Examples of antigen used: Candida albicans, tetanus toxoid, tuberculin, and fungal antigens (e.g. trichophyton and histoplasmin) Testing for Delayed Hypersensitivity (Mantoux method skin test)...4 The intradermal injection The measurement SUMMARY characteristics TYPE-I (anaphylactic) TYPE-II (cytotoxic) TYPE-III (immune complex) TYPE-IV (delayed type) Antibody IgE IgG, IgM IgG, IgM None Antigen exogenous cell surface soluble tissues & organs Response time 15-30 minutes minutes-hours 3-8 hours 48-72 hours Appearance weal & flare lysis and necrosis erythema and edema, necrosis erythema and induration Histology basophils and eosinophil antibody and complement complement and neutrophils monocytes and lymphocytes Transferred with antibody antibody antibody T-cells Examples allergic asthma, hay fever Erythroblastosis fetalis, Farmer's lung disease tuberculin test, poison ivy, granuloma AUTOIMMUNITY Autoimmune diseases are conditions in which organs or tissues are damaged as a results of the presence of autoantibody or autoreactive cells Affect 5-7% of the population and are thought to be caused by the loss or breakdown of self-tolerance Examples: Systemic Lupus Erythematosus, Rheumatoid Arthritis, Autoimmune Thyroid Diseases, Type I Diabetes Mellitus, Other diseases (Multiple Sclerosis, Myasthenia Gravis, Goodpasture’s Syndrome) EXAMPLES OF AUTOIMMUNE DISEASES Systemic Lupus Erythematosus Chronic, systemic inflammatory disease caused by immune complex formation. The word "systemic" means the disease can affect many parts of the body. Pathophysiology associated with clinical features secondary to immune complexes depositing in tissues resulting in inflammation. Parts of the body affected include: the joints, skin, kidneys, heart, lungs, blood vessels, and brain. Laboratory Diagnosis of Systemic Lupus Erythematosus (SLE)...2 Screening test for anti-nuclear antibodies (ANA) first test done. -Antibodies directed against nuclear material of cells. Flourescent anti-nuclear antibody (FANA) most widely used, extremely sensitive, low diagnostic specificity. Animal or human cells fixed to slide. Add patient serum and incubate. Wash to remove unreacted antibody. Add anti-human globulin labeled with fluorescent tag or enzyme. Laboratory Diagnosis of Systemic Lupus Erythematosus (SLE)...3 Anti-nuclear antibodies (ANA) test Patterns of reactivity: Homogenous-entire nucleus stained Peripheral-rim of nucleus stained Speckled-spots of stain throughout nucleus Nucleolar-nucleolus only stained False positives and negatives occur. Extractable Nuclear Antigen (ENA) for SLE Rheumatoid Arthritis Laboratory Diagnosis of Rheumatoid Arthritis Laboratory tests involve testing patients serum with red blood cells or latex particles coated with IgG, agglutination is a positive result. Nephelometry and ELISA techniques are available to quantitate the RF Erythrocyte Sedimentation Rate (ESR) used to monitor inflammation C-Reactive protein (CRP) is utilized to monitor inflammation Laboratory Testing of type I Diabetes Mellitus Recommendations for diagnosing diabetes state is that, patients be told they have diabetes if any of the criteria below applies: Fasting plasma glucose is above 126 mg/dl; Diabetes symptoms exist and casual plasma glucose is equal to or above 200 mg/dl; or Plasma glucose is equal to or above 200 mg/dl during an oral glucose tolerance test. If genetic predisposition is suspected perform testing to detect antibodies to pancreatic islet cells. Antibodies to insulin detected by RIA or ELISA methods. Multiple Sclerosis, MS Because the myelin is damaged, messages moving along the nerve are transmitted more slowly or not at all which slows or blocks muscle coordination, visual sensation and other nerve signals. Diagnosis of MS The basic guideline for diagnosing MS relies on two criteria: There must have been two attacks at least one month apart. An attack, also known as an exacerbation, flare, or relapse, is a sudden appearance of or worsening of an MS symptom or symptoms which lasts at least 24 hours. There must be more than one area of damage to central nervous system myelin—the sheath that surrounds and protects nerve fibers. The damage to myelin must have occurred at more than one point in time and not have been caused by any other disease that can cause demyelination or similar neurologic symptoms. Laboratory Diagnosis of MS Cerebrospinal fluid (CSF) is tested for levels of certain immune system proteins and for the presence of oligoclonal bands. These bands indicate an abnormal autoimmune response within the central nervous system, meaning the body is producing an immune response against itself. Oligoclonal bands are found in the spinal fluid of about 90-95% of people with MS, but since they are present in other diseases as well, they cannot be relied on as positive proof of MS. They may also take some years to develop. CSF Analysis for MS Myasthenia Gravis Laboratory Testing of Myasthenia Gravis Autoantibodies to the Acetylcholine receptor (AChRAb) can be detected in 80-90% of patients with myasthenia gravis. The assay measures antibodies that precipitate solublized muscle AChR that has been complexed with radiolabeled alpha- bungarotoxin (αBTX). Antibodies that bind to the receptor regions that are not sterically blocked by the αBTX are detected. Goodpasture’s Syndrome Antibodies react with antigens in the glomerular basement membrane of the kidney, results in severe necrosis. Antigen in kidney is similar to antigen found in lungs, resulting in antibody reacting with lung tissue resulting in pulmonary hemorrhage. Specific anti-basement antibodies can be demonstrated. Diagnosis Lung needle biopsy and a kidney biopsy will show immune system deposits. Kidney biopsy can also show the presence of the harmful antibodies that attack the lungs and kidneys Antiglomerular basement membrane (anti-GBM) antibody Enzyme immunoassay (EIA) Antibodies to Neutrophil Cytoplasmic Antigens (ANCA) identified by immunofluorescence Autoimmune Thyroid Diseases (Hashimoto’s Thyroiditis & Graves’ Disease) Hashimoto's Thyroiditis Hashimoto's Thyroiditis is a type of autoimmune thyroid disease in which the immune system attacks and destroys the thyroid gland. The thyroid helps set the rate of metabolism - the rate at which the body uses energy. Hashimoto’s prevents the gland from producing enough thyroid hormones for the body to work correctly. It is the most common form of Hypothyroidism (underactive thyroid). Laboratory Testing of Autoimmune Thyroid Diseases (Hashimoto’s Thyroiditis & Graves’ Disease) .....1 Laboratory Testing Hashimoto’s Thyroiditis (goitre)- hypothyroidism. The diagnosis of Hashimoto's thyroiditis is simply diagnosed by two blood tests. Routine thyroid function tests to confirm that a patient has an underactive thyroid gland. Anti-microsomal and anti-thyroglobulin antibodies are Igs which the body produces to attack specific portions of the thyroid cells which pinpoint Hashimoto's thyroiditis as the cause of the hypothyroidism. The anti-microsomal antibody test is much more sensitive than the antithyroglobulin, therefore some doctors use only the former blood test. These thyroid autoantibodies blood tests are high in about 95% of patients with Hashimoto's thyroiditis, but are not diagnostic. Laboratory Testing of Autoimmune Thyroid Diseases (Hashimoto’s Thyroiditis & Graves’ Disease) .....2 Laboratory testing of Graves’ Disease – Thyrotoxicosis Diagnosis may be straightforward, since the "classic face" with its triad of hyperthyroidism, goiter, and exophthalmos is easily recognized. Detection of the thyroid hormones T3 & T4,TSH using RIA Exophthalmos Exophthalmos, also called proptosis, is a characteristic finding in thyroid eye disease, and has been reported to occur in 34% to 93% of patients References Christine Dorresteyn Stevens, (2010). Clinical Immunology Serology A LABORATORY PERSPECTIVE 3rd Edition F.A. Davis Company1915 Arch Street Philadelphia, PA 19103 pgs 201-238 www.fadavis.com http://www.ucl.ac.uk/~regfjxe/Arthritis.htm http://www.haps.nsw.gov.au/edrsrch/edinfo/lupus.html http://pathmicro.med.sc.edu/ghaffar/tolerance2000.htm http://repro-med.net/info/cat4.php http://stemcells.nih.gov/info/scireport/chapter6.asp http://www-ermm.cbcu.cam.ac.uk/04008427h.htm http://www.biotest.de/ww/en/pub/folder_pharma/fields_of_use/autoimm une_disease.htm http://72.14.203.104/search?q=cache:H7KcpVQ4xkYJ:www.peppypaws.c om/Glossary.html+Forbidden+clone+theory&hl=en&client=firefox-a Thanks for listening & contributing