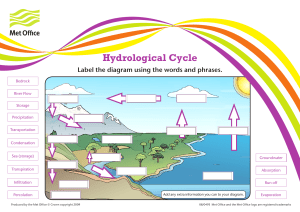
Acknowledgment It gives me great pleasure to express my gratitude towards our chemistry teacher Mrs. for her guidance, support and encouragement throughout the duration of the project. Without her motivation and help the successful completion of this project would not have been possible. Student Index 1. Acknowledgment 2. Objective Of The Project 3. Introduction 4. Theory 5. Experimental Procedure ● Experiment 1 ● Experiment 2 ● Experiment 3 6. Bibliography Objective of the Project The objective of the project is comparison of rate of evaporation of different liquids (acetone, benzene and chloroform). Also to demonstrate how the rate of evaporation depends on following factors: i) Surface Area of Liquid: As surface area of liquid increases the rate of evaporation of the liquid increases ii) Temperature of Surroundings: As the temperature of surroundings increases randomness in molecules increases and the rate of evaporation increases iii) Air Current: The rate of evaporation is higher in the presence of air current. Introduction When a liquid is placed in an open vessel, it slowly escapes into gaseous phase eventually leaving the vessel empty. This phenomenon is known as vaporization or evaporation. Evaporation of liquids can be explained in the terms of kinetic molecular model although there are strong molecular attractive forces which hold molecules together. The molecules having sufficient kinetic energy can escape into gaseous phase. If such molecules happen to come near the surface in a sample of liquid all the molecules do not have same kinetic energy. There is a small fraction of molecules which have enough kinetic energy to overcome the attractive forces and escapes into gaseous phase. Evaporation causes cooling. This is due to the reason that the molecules which undergo evaporation have high kinetic energy therefore the kinetic energy of the molecules which are left behind is less. Since the remaining molecules which are left have lower average kinetic energy. Therefore temperature is kept constant the remaining liquid will have same distribution of the molecular kinetic energy and high molecular energy will kept one escaping from liquid into gaseous phase of the liquid is taken in an open vessel evaporation will continue until whole of the liquid evaporates. Theory Factors affecting the rate of evaporation: (1) Nature of Liquids: The magnitude of inter-molecular forces of attraction in a liquid determines the speed of evaporation. Weaker the inter-molecular forces of attraction, larger are the extent of evaporation. In diethyl ether rate of evaporation is greater than that of ethyl alcohol. (2) Temperature: The rate of evaporation of liquids varies directly with temperature. With the increase in the temperature, fraction of molecules having sufficient kinetic energy to escape out from the surface also increases. Thus with the increase in temperature rate of evaporation also increases. (3) Surface Area: Molecules that escape the surface of the liquids constitute the evaporation. Therefore larger surface area contributes accelerating evaporation. (4) Composition of Environment: The rate of evaporation of liquids depends upon the flow of air currents above the surface of the liquid. Air current flowing over the surface of the liquid took away the molecules of the substance in vapour state there by preventing condensation. Experiment No. 1 Aim: To compare the rates of evaporation of acetone, benzene and chloroform. Requirement: Three same size Petri dishes of diameter 10 cm, 10 ml. pipettes, stop watch, acetone benzene and chloroform. Procedure: 1. Clean and dry all Petri dishes and identify them as A, B and C. 2. Pipette out of 10 ml. acetone in Petri dish "A" with stopper similarly pipette out of 10 ml. of benzene and chloroform in each of Petri "B" and "C". 3. Remove the cover plates from all Petri dishes and start the stop watch. 4. Let the Petri dishes remain exposed for 10 minute. Now cover each of the petri dish and note the volume of remaining material in them. Observation: Liquid Volume Volume Petri Taken Remaining Evaporated dish (V1) ml. (V2) ml V=V1–V2 Rate (V/T) ml/s 8/600= A 10 2 8 0.0133 7/600= B 10 3 7 0.0116 6/600= C 10 4 6 0.010 Results: Rate of evaporation of Acetone is 0.0133 ml/s. Rate of evaporation of Benzene is 0.0166 ml/s. Rate of evaporation of Chloroform is 0.010 ml/s. Experiment No. 2 Aim: To study the effect of surface area on the rate of evaporation of diethylether. Requirement: Three Petri dishes of diameter 2.5 cm., 5 cm., and 7.5 cm. with cover, 10 ml. of pipette and stop watch. Procedure: 1. Clean and dry all Petri dishes and mark them as A, B and C. 2. Pipette out of 10 ml. diethylether in each of the Petri dishes A, B and C and cover them immediately. 3. Uncover all three Petri dishes and start the stop watch. 4. Note the remaining volume after 10 min. vaporization of diethyl ether from each Petri dish. Observation: Petri Volume Diameter Remaining Evaporated Dishes Taken of P.T.Ds. Vol. (ml.) volume Marked (ml.) A 2.5 10 4 6 B 5.0 10 2 8 C 7.5 10 0 10 Results: The order of rate evaporation of acetone in three petri dishes according to their diameter is 7.5 > 5.0 > 2.5 cm. Experiment No.3 Aim: To study the effect of temperature on the rate of evaporation of acetone. Requirement: Two Petri dishes of 5 cm. diameter each stop watch, 10 ml. pipette, thermometer, and thermostat. Procedure: 1. Wash and Clean, dry the Petri dishes and mark them as A, B. 2. Pipette out of 10 ml. of acetone to each of Petri dishes A and B and cover them. 3. Put one Petri dish at room temperature and to the other heat for same time. 4. Note the reading. Observation: Petri dishes Marked A B Volume Evaporated Time Temperature Taken volume (Sec.) (K) (ml.) (ml.) 10 303 10 10 20 313 10 10 Results: The order of evaporation of acetone in two Petri dishes as given Room Temperature < Heating. Experiment No.4 Aim: To study the effect of air current on the rate of evaporation of acetone. Requirement: Two Petri dishes acetone. Procedure: 1. Clean and dry the Petri dishes and mark them as A and B. 2. Keep one dish where no air current and other under a fast air current. 3. Note the reading. Observation: Petri Time dishes Conditions (Sec.) Marked A With fan 40 B Without fan 50 volume Evaporated (ml.) 10 10 Results: Rate of evaporation with fan = 0.25 ml/sec Rate of evaporation without fan = 0.20 ml/sec Interpretation of Result i) Comparison of rate of evaporation of acetone, benzene and chloroform: Rate of evaporation of acetone, benzene and chloroform is in the order: Chloroform < Benzene < Acetone Hence the intermolecular forces are in the order: Chloroform > Benzene > Acetone. ii) Effect of area on rate of evaporation: Larger the surface area more is evaporation, as it is observed that acetone in petri dish with larger surface area evaporates at a faster rate. iii) Effect of temperature on rate of evaporation: Observation clearly shows that the evaporation increases with temperature. So an increase in temperature favours the rate of evaporation. iv) Effect of air current on rate of evaporation: In the presence of fan the rate of evaporation is found to be more, hence the rate of evaporation of liquid increases with the increase in rate of flow of air current. Bibliography i) en.wikipedia.org ii) practicalchemistry.org iii) google.com iv) N.C.E.R.T. Notebook For Chemistry, Class XII