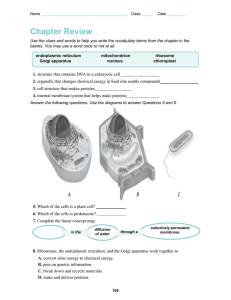
La Salle University Ozamiz City SY 2022-2023 Second Semester College of Arts and Sciences Science Department Biochemistry Laboratory Manual Ms. Kissy Jean Paredes Instructor TABLE OF CONTENTS TITLE PAGE 1 LABORATORY ACTIVITY 1 Laboratory Apparatuses and Techniques 3 EXPERIMENT 1 The Microscope and Cells 5 EXPERIMENT 2 Water: Its Properties and Purification & Preparation of Buffers 7 EXPERIMENT 3 Isolation and Purification of Proteins & Denaturation of proteins 10 EXPERIMENT 4 Color Reaction for Amino Acids and Proteins 14 EXPERIMENT 5 Analysis of Carbohydrates 18 EXPERIMENT 6 Test for Lipids 20 EXPERIMENT 7 Nucleic Acids: DNA and RNA 23 EXPERIMENT 8 Qualitative Analysis of Urine 26 Activity No. 1: Laboratory Apparatus & Techniques A. Safety in the Laboratory The concern for laboratory safety can never be overemphasized. You must always be aware that chemicals used in the laboratory are potentially toxic, irritating, and flammable. However, such chemicals are a hazard only when they are mishandled or improperly disposed of. It is my experience as a lab instructor for 17 years that accidents happen least often to those who come to each lab session mentally prepared and with a complete understanding of the experimental procedures to be followed. Because dangerous situations can develop unexpectedly, though, you must be familiar with general safety practices, facilities, and emergency actions. Carelessness on the part of one person can often cause injury to others. As an adult, please be advised that you must be accountable. Ask the elderly, if possible, to help you. Keep in mind and always observe the following: 1. Use your common sense. 2. Read the laboratory instructions/procedures carefully before starting an experiment. 3. Never perform unassigned experiments or work alone. Somebody older must be present when working on the experiments. Never work with a child. 4. Never play, eat, drink, or smoke while working. 5. Read the labels of bottles twice to be sure you use the right chemical for the experiment. Don’t worry. Such chemicals are just in your home. 6. Always follow instructions. 7. Wear safety goggles/face shields, laboratory gowns/aprons, and gloves when doing any experiments. 8. Always wear shoes to protect your feet from chemical spills. 9. Please be very careful in using open flames. 10. Keep all books and none essential items away from the working area. 11. Tie back long hair and restrict any loose clothing. 12. Never use broken or cracked glassware. 13. Report all injuries or accidents to somebody elder than you immediately. 14. Dispose of any waste materials in appropriate containers. 15. Close gas cocks, faucets, and switch off the light and ceiling fans before leaving your make-shift laboratory room. 16. Wash your hands after every experiment. 17. Always keep your working area clean and dry. B. FIRST AID IN THE LABORATORY The following are common laboratory accidents that need first aid treatments: 1. Chemicals in the eye. Hold an eye open and immediately flush with water. Continue for at least five minutes. 2. Chemical spilled on skin. Flush with a large amount of water for five to twenty minutes. 3. Severe bleeding. Apply pressure or tourniquet. 4. Minor burns. Apply burn ointment. To reiterate, please use your common sense. Be vigilant and follow these safety measures for a successful laboratory experiment at home. C. HAZARDS IN THE LABORATORY – Important Terms • Severe Toxicity- adverse effects of a substance that result either from a single exposure or multiple exposures in a short space of time (usually less than 24 hours) • Irritant – causes redness, inflammation • Corrosive – “eats away” tissue gradually • Carcinogenic – causes cancer • Flammable –easily set on fire • Biohazard –substances that pose a threat to the health of living organisms, primarily that of humans. D. Personal Protective Equipment (PPE) Personal Protective Equipment (PPE) refers collectively to equipment such as safety goggles, lab coats, gloves, protective shoes, respiratory protective equipment, ear defenders, and similar equipment used to protect the person during their work. Laboratory Apparatus and Techniques View the videos below to know the apparatus and techniques used in the laboratory: Process Questions: 1. What is the importance of knowing different laboratory techniques? 2. Give at least three(3) potential chemical hazards in the lab. Experiment 1 The Microscope and Cells I. Introduction II. Objectives At the end of the experiment, the students should be able to: (refer to the learning outcomes) 1. 2. III. Apparatus& Chemicals IV. Procedures: V. Data & Results A. Parts of the compound microscope Write the correct label for each part of the microscope shown below: B. Viewing Specimen under the Microscope Specimen 1. Cut letter “e” 2. Transparent ruler 3. Thread 4. Bacteria Description Sketch the Specimens at low and high power 5. Cheek cells 6. Section tissue of lung VI. Analysis (Discussion) Discuss the concepts of how to use microscopes and how they work. VII. QUESTIONS 1. Refer to Part V. A; In tabulated form, list the parts of the microscope and give their functions/uses. 2. What objective lens you should have in place to begin looking at your specimen? Explain why. 3. What happens if you try to use the coarse adjustment when the 10X lens is in place? 4. How would you compare the 4x and 10x objectives in terms of differences in magnification and resolution?? 5. Assuming the light is on and the oculars are in place, what are the next 2 steps? 6. Give at least two precautionary measures in the use of the microscope. VIII. Conclusion IX. References X. Documentation Experiment 2 Water: Its Properties and Purification & Preparation of Buffers I. Introduction II. Objectives At the end of the experiment, the students should be able to: (refer to the learning outcomes) 1. 2. III. Apparatus& Chemicals IV. Procedures: V. Data & Results I. Water: Its Properties and Purification A. Properties of Water Properties Observation Cohesion Hydrates Pentahydrate) (Copper Sulfate B. Impurities in seawater Impurities 1. Sodium ion (Na+) 2. Chloride ion (Cl-) Observation C. Methods of purifying impure water Methods Observation 1. Sedimentation 2.Decantation 3.Filtration 4.Distillation D. Hardness of water Descriptions: II. Preparation of Buffers A. Descriptions (Remarks) 1. pH and volume of buffer chosen: 2.Give the formula of the acid and salt used to prepare the buffer solution 3.Calculation of the salt/acid ratio of the buffer: 4. What is the pH of the buffer solution used using the pH meter? 5. Is the experimental pH gives greater than, lesser than, or equal to the desired pH? 6. If the experimental pH obtained is greater than the desired pH, what substance (acid or salt) is added to adjust the experimental pH to the desired pH? B. Effect of Buffer Remarks Give the pH changes before and after putting acid or base to the buffer solution VI. Analysis (Discussion) In the first section, discuss the properties and purification of water, and in the 2nd section discuss the concepts on the preparation of buffers. VII. QUESTIONS Answer questions 1 -4 based on your experimentation at home. 1. How does it differ? 2. What variations do coins have that allow one to hold more or less than the other? 3. Do all pennies hold the same amount of water? Explain 4. What other things besides size affect the number of drops? 5. Why do you think rainwater is soft Qs 6-10, are for buffers: 6. Identify the two components of an acidic buffer solution and explain the function of each component. Give also the criteria of a classic buffer system: acidic buffer or alkaline buffer. 7. Write the chemical equilibrium equation representing the sodium phosphate buffer system. 8. Explain how water reacts when acid is added, and when the base is added. 9. Explain how one component of a buffer system reacts when acid is added, and the other component reacts when the base is added. 10. Based on the experiment, what can you say about buffers and pH changes? VIII. Conclusion IX. References X. Documentation Experiment 3 Isolation and Purification of Proteins & II) Denaturation of Proteins I. Introduction II. Objectives At the end of the experiment, the students must have: (refer to the learning outcomes) 1. 2. III. Apparatus& Chemicals IV. Procedures: V. Data & Results I. Isolation and Purification of Casein and Albumin A. Casein Isolation Appearance before isolation Appearance after isolation B. Albumin Isolation Appearance before isolation Appearance after isolation II. Denaturation of proteins A. Experiment at Home Sample Added Observation 1 Heat 2 NaCl 3 NaHCO3 4 Lemon juice or Vinegar 5 Rubbing alcohol 6 Tap water 7 AgNO3 B. Experiment in the Lab 1. Heat effect Albumin Casein Saliva 1 2 2. pH Effects Effect Albumin Medium (pH) Water (control) (pH 7) 10 % NaOH (pH 14) on Effect on Casein Semen Food sample 5 % NaHCO3 (pH 9) 2% HCl (pH 2) 3. Effects of ethanol, lead (II) nitrate, silver nitrate, and tannic acid Agent Effect on Albumin Effect on Casein None (Control) 95 % ethanol Pb2+ Ag+ Tannic acid VI. Analysis (Discussion) Discuss the principles of isolation of proteins and denaturation of proteins. VII. QUESTIONS 1. Of the methods you tested, which would be more likely to be used in the food industry? 2. In your own words, explain the effect of heat on the solubility of albumin. 3. Is casein more soluble in base or in acid? Why? 4. When milk sours, lactic acid is produced, and a white precipitate forms. What is this precipitate? Explain what is happening. 5. Did you observe any differences in the solubility of casein and albumin at pH 2? at pH 9? at pH 14? From your observations, which is more affected by pH, casein or albumin? 6. What kinds of disruptions to the native conformation occur in each of the following? In other words, are hydrogen bonds, disulfide linkages, etc. disrupted? Albumin is heated. Alcohol is added to egg white. Metal ions are added to albumin. VIII. Conclusion IX. References X. Documentation Experiment 4 Color Reactions of Amino Acids & Proteins I. Introduction II. Objectives At the end of the experiment, the students should be able to: (refer to the learning outcomes) 1. 2. III. Apparatus& Chemicals IV. Procedures: V. Data & Results Color Reactions for Amino Acids & Proteins 1. Biuret Test Samples Color of solution the original Color after NaOH/CuSO4 (Biuret reagent) 2. Ninhydrin Test Samples Color of original Color with Ninhydrin Color after solution reagent) minutes 10 3. Xanthoproteic Test Color before adding NaOH Color NaOH Color Observation Remarks Samples 4. Millon’s Test Samples after adding 5. Hopkin’s Cole Test Samples Color Observation Remarks 6. Sakaguchi’s Test Samples Color Observation Remarks 7. Sulfur Test Samples Color Observation Remarks VI. Analysis (Discussion) Discuss the principles of the color test for amino acids and proteins. VII. QUESTIONS: 1. Write the reactions involved in each test. 2. Do all protein samples react positively with the Biuret test? Justify your answer. 3. What is a peptide? State the psychological significance of breaking down proteins into peptide. VIII. Conclusion IX. References X. Documentation Experiment 5 Qualitative Test for Carbohydrates I. Introduction II. Objectives At the end of the experiment, the students should be able to: (refer to the learning outcomes) 1. 2. III. Apparatus& Chemicals IV. Procedures: V. Data & Results (Note: Indicate the color of solution or precipitate and include (+)/(-) for the results) A. General Test for Carbohydrates Sample Solutions Solubility Molisch’s Test B. Iodine Test for Polysaccharides Sample Solutions Food Samples · Dairy Color Response to Iodine · Fruit juice · Cereals · Crackers · Cookies C. Qualitative Test for Sugars Sample Solutions Benedict’s Barfoed’s Seliwanoff’s Bial’s · VI. Analysis Discuss the principle involved in each test for carbohydrates. VII. Answers to Questions 1. Give at least three clinical correlations to the chemical test for sugars. 2. Compare the results you obtained for the iodine test of starch to the iodine test of hydrolyzed starch. Explain your results. 3. What is meant by the term “reducing sugar”? 4. What is the purpose of testing water in the specific test for sugars? 5. An unknown carbohydrate gave a purple junction when tested with the Molisch reagent, turned red when reacted with Seliwanoff’s reagent, and quickly gave a red precipitate when reacted with Barfoed’s reagent. What conclusions can be made about this carbohydrate? 6. What test could be used to differentiate between sucrose and lactose? glucose and starch? glucose and fructose? Explain. VIII. Conclusion IX. References X. Documentation Experiment 6 Tests for Lipids I. Introduction II. Objectives At the end of the experiment, the students must have: (refer to the learning outcomes) 1. 2. III. Apparatus& Chemicals IV. Procedures: V. Data & Results (Note: Indicate the color of solution or precipitate and include (+)/(-) for the results) Part I. Qualitative Analysis of Lipids I. Physical Properties A. Solubility Use the letter ‘S’ for soluble, ‘PS’ for partially soluble, and ‘I’ for insoluble. Solubility Solubility in Water in Organic Solvent (Chloroform) Samples B. Transparency Test Samples Translucent Spot Result Remarks Part II. Identifying Lipids Using Chemical tests Samples: Tests A. Acrolein B. Unsaturation C. Rancidity D. Saponification E. Cholesterol 1. Liebermann–Burchard 2. Salkowski Part III. 1. Mass of a dry and clean 125-mL Erlenmeyer =_____________________g 2. Mass of the 125-mL Erlenmeyer flask plus potato =_____________________g 3. Mass of potato chips = #2 - #1 =_____________________g flask chips 4. Mass of dry and clean 150-mL beaker =_____________________g 5. Mass of the 150-mL beaker plus lipid extracted =_____________________g 6. Mass of lipid extracted = #5 - #4 =_____________________g 7. % of lipid in potato chips = (mass of lipid extracted / mass of chips) x 100 = (#6 / #3) x 100 = _____________% = Experimental value 8. Calculate the % of lipid in potato chips from the Nutritional label = (grams of fat per serving/ serving size in grams) x 100 = _____________%= Theoretical value 9. Calculate the % error = (Difference between theoretical and experimental values/ theoretical value) x 100 = (Difference between step-7 and step-8÷step-8) x 100=_______% VI. Analysis Discuss the principle involved in each test of lipids. VII. Answers to Questions 1. What do “LDL” and “HDL” stands for? Explain their importance in biological systems. 2. Show the chemical reaction between acetic anhydride and cholesterol. 3. How can you tell that the dark wet spot on the paper towel is fat and not water? 4. Rank from most to least the percentage of lipid extracted from your food samples (if there’s any). Look at the Nutrition Facts label on the packages of all the foods and rank them. Did your ranking agree with the ranking of the product labels? VIII. Conclusion IX. References X. Documentation Experiment 7 Nucleic Acids: DNA & RNA I. Introduction II. Objectives At the end of the experiment, the students should be able to: (refer to the learning outcomes) 1. 2. III. Apparatus& Chemicals IV. Procedures: V. Data & Results (Note: Indicate the color of solution or precipitate and include (+)/(-) for the results) I. Extraction of DNA from cheek cells Description Extracted DNA II. Isolated RNA from yeast Theoretical results Experimental results RNA yeast from (filtrate) III. Qualitative Tests for Nucleic Acids Tests Results Solubility: Test for Nucleoproteins: Mild Acid Hydrolysis: A. Test for the presence of Purines: B. Test for the presence of Pentose: C. Test for the presence of Phosphate: VI. Analysis Discuss the principle involved in the extraction and isolation of DNA & RNA. VII. Answers to Questions 1.Why are we using dishwashing liquid? What does it do to the cells? 2.Why did we use the pineapple juice? 3. Why did the isopropyl alcohol need to be cold? 4. What role does salt play? 5. What is the purpose of using 10% NaOH in RNA hydrolysis? VIII. Conclusion IX. References X. Documentation Experiment 8 Qualitative Analysis of Urine (Urinalysis) I. Introduction II. Objectives At the end of the experiment, the students should be able to: (refer to the learning outcomes) 1. 2. III. Apparatus& Chemicals IV. Procedures: V. Data & Results A. Physical Properties of Urine Physical Properties Urine Sample Color Appearance (Turbidity) Odor pH Specific gravity (state the normal values) B. Normal Organic/Inorganic Constituents (Note: Indicate the color of solution or precipitate and include (+)/(-) for the results) Tests Results 1. Urea 2. Uric Acid 3. Creatinine 4. Chloride 5. Phosphate C. Pathological (Abnormal) Organic Constituents Urine Sample Tests Color 1. Ketone 2. Glucose 3. Albumin 4. A. Pigment Bile a. Gmelin’s b. Fouchets Test B. Bile Acids and Bile Salt a. Pettenkofer's b. Hays 5. Blood Unknown Urine Sample (+ or -) Color (+ or -) VI. Analysis Discuss the principle involved in urinalysis. VII. Answers to Questions 1. Why it is important to refrigerate a urine sample if the analysis cannot be performed immediately after collecting it? 2. Examine the following urinalysis data from Patient's A and B and answer questions that follow. Normal range Patient A Patient B color pale yellow dark yellow medium yellow appearance clear cloudy clear pH 4.5 - 8.0 6.0 7.2 specific gravity 1.001 - 1.030 1.015 1.03 blood none +++ none sodium 40-220 mEq/L 340 mEq/L 140 mEq/L glucose none none none ketone bodies none none none creatinine 150 mg/dL 180 mg/dL 150 mg/dL ammonia 50 - 70 mg/dL 90 mg/dL 65 mg/dL Patient A is a 45-year-old man that came to his physician after spending a second sleepless night with excruciating lower abdominal pain. The pain seemed to come in waves and was unrelieved by analgesics or lying or standing in any position. He had not experienced any similar pain before. On physical examination, there are no abnormal findings. 3. What abnormal findings are present on Patient A's urinalysis chart? 4. Based on these abnormalities what do you think could be the reason for Patient A's pain? 5. Can you observe anything abnormal in Patient B's urinalysis chart? VIII. Conclusion IX. References X. Documentation