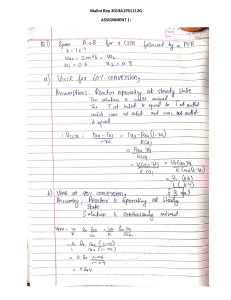Decomposition Type Analysis of Continuous Reactors ▪ PFR: Liquid Reactions For reactant A: VPFR = Lecture No. 6.1 A PLUG FLOW REACTORS (PFR) or Tubular Reactors (TR) dFA FAo r A FA VPFR = vo k A C A o n −1 VPFR = For n=1 A. Constant Volume ( A=0, for liquids) B. Variable Volume ( A 0, for gases) For n 1 Ch.E. 2115 Lola Domnina B. Pestaňo, Ph.D. XA 0 dX A (1 − X A )n VPFR = =0 dCA −k A CA n CA CAo PFR vo − ln (1 − X A ) kA = PFR vo 1 −1 k A ( n − 1) CAo n −1 (1 − X A )n −1 = 1 1 −1 k A ( n − 1) CAo n −1 (1 − X A )n −1 Fj dFj Fjo rj VPFR = v o 1 k A C A o n −1 VPFR = PFR 1 VPFR = v o A = XA 0 XA 0 −CAodX A −k A CA n dX A (1 − X A )n 1 − ln (1 − X A ) kA NOTE: The space time equations are the same as the time formulas for CV Batch Reactors 2 @UST Ch.E. Department 2 Problems Decomposition Type ▪ PFR: Gaseous Reactions VPFR = 0 A Fj dFj Fjo rj PS 3A For reactant A: VPFR = FA FAo dFA rA VPFR = v o VPFR = v o PFR = dCA −k A CA n VPFR = v o −CAodX A XA o NAo (1 − X A ) Vo (1 + A X A ) −k A VPFR = CA CAo v oCAo k A CAon 1 k A CAon −1 XA o XA o 1 + A XA 1 − XA 1 + A XA 1 − XA XA 0 −CAodX A −k A CA n For Variable Volume (Constant Pressure) gas reactions: V=Vo(1+ AXA) n PS 3B n dX A (variable density) Solved numerically n PS 3C dX A For gas reactions 3 @UST Ch.E. Department 3 3.Repeat Problems 1 and 2 if the decomposition is now 2nd order and half-life from batch reactor data is still 30 min for the same CAo in all cases. 4.Given the gas phase elementary reaction: 2A → R with a feed that is 80%A, 20% inerts. If batch data at constant pressure gave a half-life of 30 min, what space time will be needed in a PFR to attain 80% conversion of A? (Answer: 80.15mins) 5.Repeat 4 if the reaction is first order. (Answer: 55.65mins) 6.Repeat 4 and 5 to determine the % conversion in the PFR if the space time is to be 2 hours. (Answer: 0.8691, 0.98105) 4 @UST Ch.E. Department 4 Problem 4 4.Given the gas phase elementary reaction: 2A → R with a feed that is 80%A, 20% inerts. If batch data at constant pressure gave a half-life of 30 min, what space time will be needed in a PFR to attain 80% conversion of A? (Answer: 80.15mins) Given: gas phase elem. reaction: 2A → R n = 2 yAo=0.8 yIo=0.2 Constant Pressure (VV) Batch reactor: t1/2 = 30 mins 5 @UST ChE Department Problem 4 Required: PFR: =? if XA=0.8 Required: PFR: =? if XA=0.8 4.Given the gas phase Solution: elementary reaction: 0.0292 vol For n=2, 2A → P: k A = 2A → R with a feed that CA mole − min. PFR: Gas Phase is 80%A, 20% inerts. If n batch data at constant X 1 + A XA 1 dX A pressure gave a half-life PFR = k A CAon −1 o 1 − XA of 30 min, what space 2 time will be needed in a X 1 + A XA 1 dX A PFR to attain 80% PFR = k A CAo o 1 − XA conversion of A? when XA=0.8 PFR=? (Answer: 80.15mins) 2 Given: 0.8 1 − 0.4X 1 A dX A gas phase elem. reaction: PFR = 0 0.0292 1 − XA CAo 2A → R n = 2 CAo yAo=0.8 yIo=0.2 Constant Pressure (VV) PFR = 80.15 mins. Batch reactor: t1/2 = 30 mins 6 Solution: Detm. kA from Constant Pressure (or VV) Batch data: o For n 1, VVBR decomposition: t= 1 k A CnAo−1 XA X A )n −1dX A (1 − X A )n (1 + A 0 V = Vo A A XA A For: 2A → R: A = A = (1 − 2) / 2 = −0.5 y AO = −0.5(0.8) = −0.4 1 kA = 30 * C Ao kA = A 0.5 0 (1 − 0.4X A )dX A (1 − X A )2 0.0292 vol CAo mole − min. 5 @UST Ch.E. Department @UST Ch.E. Department 6 1 At the end of this topic, you should be able to: Analysis of Continuous Reactors ▪ Understand the concept of space time ( ) and space velocity (SV) for continuous reactors. ▪ Use analytical or numerical methods to solve the steady-state applications for: ▪ Constant Stirred Tank Reactor (CSTR) or Mixed Flow Reactor (MFR) ▪ Plug Flow Reactor (PFR) or Tubular Reactor (TR) ▪ Combination of CSTR and PFR Lecture No. 6A A. Reactors in Series B. Space Time C. Numerical and Analytical Method Ch.E. 2115 Lola Domnina B. Pestaňo, Ph.D. @UST Ch.E. Department @UST Ch.E. Department 1 2 2 Review of Continuous Reactors Review of Continuous Reactors MFR or CSTR TR or PFR Reactants are continuously fed to a tube so that what goes in first also comes out first. Reaction proceeds across the reactor length and concentrations vary from entrance to exit as there is no lateral mixing. Radial concentrations are however assumed constant. MFR or CSTR Reactants are continuously fed to a well mixed tank and the products are also withdrawn continuously. At steady state, the concentration of any material in the tank is also the same as that in the product stream. @UST Ch.E. Department 3 3 @UST Ch.E. Department 4 4 MFR or CSTR Plug Flow Reactor (PFR) Steady State Flow: No Spatial Variation: 5 UST Chemical Engineering Dept. 5 @UST Ch.E. Department 6 6 1 Plug Flow Reactor (PFR) Summary of Mole Balances FA = C A v 7 UST Chemical Engineering Dept. 7 @UST Ch.E. Department8 8 Reactors in Series • reactors are connected together so that the exit stream of one reactor is the feed stream for another reactor. FA 0 XA0 = 0 I. Reactors in Series FA1 , X A1 V1 V2 X Ai = FA3 , X A3 V3 FA 2 , X A 2 total number of moles of A reacted up to pt. i mole of A fed into the first reactor 10 @UST Ch.E. Department 9 10 FA 0 V1 XA0 = 0 FA1 , X A1 FA 0 XA0 = 0 FA1 , X A1 V1 V2 V2 FA3 , X A3 V3 Consider the figure above, X Ai = FA 2 , X A 2 Entering Molar rate of A Considering the individual reactors above, FA1 Reactor 1: FA1 = FA0 − FA0 X A1 Reactor 2: FA 2 = FA0 − FA0 X A 2 11 FA 2 , X A 2 Consider the CSTR: total number of moles of A reacted up to pt. i mole of A fed into the first reactor Reactor 3: FA3 = FA0 − FA0 X A3 FA3 , X A3 V3 Rearranging, 11 @UST Ch.E. Department - Exit Molar rate of A + Rate of generation of A =0 - FA 2 + rA 2 V2 =0 V2 = FA1 − FA 2 -rA 2 12 @UST Ch.E. Department 12 2 Space Time ( ) II. Space Time, Space time, : the time necessary to process one reactor volume of fluid based on entrance conditions. and = Space Velocity, SV V vo vo = entering volumetric flow rate Space velocity (SV): number of reactor volumes of feed at specified conditions which can be treated in unit time SV = vo 1 = V 14 @UST Ch.E. Department 13 14 Space Time ( ) Space Time ( ) 20 m 20 m Fluid directly upstream the reactor Example: = 2 min A space time of 2 minutes means that every 2 min one reactor volume of feed at specified conditions is being treated by the reactor. REACTOR * space time is also referred to as “holding time” or “mean residence time” Example: SV = 5/hr * considering the figure above: the time it takes for the fluid to enter the reactor Thus, a space-velocity of 5 hr-1 means that five reactor volumes of feed at specified conditions are being fed into the reactor per hour. completely is referred to as the space time. 15 @UST Ch.E. Department 15 16 @UST Ch.E. Department 16 Covered Applications Determination of Reactor Volume and/or Space time ▪ Single MFR or CSTR III. Numerical and Analytical ▪ Equivalent PFR ▪ Combination of Reactors Method Determination of Over-all Conversion ▪ Single MFR or CSTR ▪ Equivalent PFR ▪ Combination of Reactors Determination of Reaction Kinetics ▪ Unknown Order and Rate Constant 18 UST Chemical Engineering Dept. 17 18 3 Decomposition Type (aA → P, -rA=kACAn) Decomposition Type (aA → P, -rA=kACAn) ▪ Single CSTR ▪ Multiple CSTRs in Series V = v o ( C jo - C j ) V = - rj V= - rj = F jo - F j C jo - C j C = - rj jo = C j - rj = F jo X j Vm = - rj v o (C jo -C j ) V= kC jn C jo - C j kC j C jo = C j + kC Vm = Xj = n vo Xj kC jo n-1(1-X j )n k C jo n -1 m (1 - X j ) n = v o ( C jm −1 - C jm ) - r jm m - r jm jm − 1 = C jm 19 @UST Ch.E. Department 19 m = = Fjm −1X jm -rjm v o ( C jm −1 - C jm ) k m C jm n C jm −1 - C jm k m C jm C jm − 1 τm = n = C Vm = jm + kmC v o C Ao (X jm - X jm -1 ) k m C jo n (1 - X jm )n C jo (X jm - X jm -1) k m C jo n (1 - X jm )n n jm m 20 @UST Ch.E. Department 20 Decomposition Type (aA → P, Problems -rA=kACAn) 1. A first order liquid decomposition gave a half-life of 30 min in a batch reactor. What total space time will be needed to attain 80% conversion in: a. One CSTR (Answer: 173.16 mins) b. A PFR (Answer: 69.67 mins) c. Two equal sized CSTRs (Answer: 107.02mins, where XA1 = 0.5528) d. Equal sized CSTR and PFR in series (Answer: 81.76mins, where XA1 = 0.4857) e. Equal sized PFR and CSTR in series (Answer: 81.76mins, where XA1 = 0.6111) ▪ Single MFR or CSTR Fjo -Fj V= -rj = Fjo X j V = -rj v o ( C jo - C j ) - rj C jo - C j = - rj ▪ Multiple MFR or CSTR in Series Vm = Fjm −1-Fjm -rjm = Fjo (X jm − X jm −1 ) Vm = -rjm v o ( C jm −1 - C jm ) - r jm C jm −1 - C jm = m - r jm ▪ Single TR or PFR V= Fj dFj Fjo rj V = vo Cj dC j C jo rj = Cj dC j C jo rj C jm dC j C jm−1 rj = XA −C j0 dX j rj 0 ▪ TR or PFR in series Vm = Fjm dFj Fjm−1 rj Vm = v o C jm dC j C jm−1 rj m = m = X jm −C j0 dX j X jm−1 rj 21 @UST Ch.E. Department 21 22 @UST Ch.E. Department 22 Problems Problems 2. A first order liquid decomposition gave a half-life of 30 min in a batch reactor. What % over-all conversion is attained in a. One CSTR, space time of 1 hour(Answer: 58%) b. A PFR, space time of 1 hour (Answer: 75%) c. Two equal sized CSTRs, each with a space time of 30 min (Answer: 65.11% where XA1 = 0.41) d. CSTR and PFR in series, each with a space time of 30 min (Answer: 70.5% where XA1=0.41) e. PFR and CSTR in series, each with a space time of 30 min. (Answer:70.5% where XA1 =0.5) 3.Repeat Problems 1 and 2 if the decomposition is now 2nd order and half-life from batch reactor data is still 30 min for the same CAo in all cases. 4.Given the gas phase elementary reaction: 2A → R with a feed that is 80%A, 20% inerts. If batch data at constant pressure gave a half-life of 30 min, what space time will be needed in a PFR to attain 80% conversion of A? (Answer: 80.15mins) 5.Repeat 4 if the reaction is first order. (Answer: 55.65mins) 6.Repeat 4 and 5 to determine the % conversion in the PFR if the space time is to be 2 hours. (Answer: 0.8691, 0.98105) PS 3A PS 3B PS 3C 23 @UST Ch.E. Department 23 - rm j -rjm Vm = C jm −1 - C jm C n j Fjm −1-Fjm 24 @UST Ch.E. Department 24 4 At the end of this topic, you should be able to: ▪ Apply the mole balance and its applications to steady state: ▪ Constant Stirred Tank Reactor (CSTR) or Mixed Flow Reactor (MFR) ▪ Plug Flow Reactor (PFR) or Tubular Reactor (TR) ▪ Combination of CSTR and PFR ▪ Use both graphical and numerical or analytical methods to solve the applications Analysis of Continuous Reactors Lecture No. 6B Graphical Method Ch.E. 2115 Lola Domnina B. Pestaňo, Ph.D. @UST ChE Department 1 2 Covered Applications Common Nomenclatures Determination of Reactor Volume ▪FJo = input molar rate of specie J = voCJo ▪FJf = output molar rate of specie J = vfCJf ▪vo, vf = volumetric feed & exit rates ▪Cjo, CJf = inlet & outlet concentrations ▪-rJ = rate of reaction of J ▪XJ = (FJo – FJf)/FJo = fract. conv. of J ▪τ = Space Time = time to process one reactor volume of feed = V/vo ▪V = reactor volume ▪θ = residence time = V/vf ▪ Single MFR or CSTR ▪ Equivalent PFR ▪ Combination of Reactors Determination of Over-all Conversion ▪ Single MFR or CSTR ▪ Equivalent PFR ▪ Combination of Reactors Determination of Reaction Kinetics ▪ Unknown Order and Rate Constant 3 4 @UST ChE Department 3 @UST ChE Department 4 Solution Methods Graphical Method ▪ Given the following –rA and CA data: ▪ Graphical ▪ ▪ Applicable if data of –rJ and CJ or XJ are available Uses: ▪ Levenspiel plots - Plot of 1/-rJ vs CJ or XJ ▪ or Walas Plot - Plot of -rJ vs CJ CAi -rAi ▪ Numerical or Algebraic Methods ▪ Applicable if the kinetics of the reaction is known or unknown, meaning the order, rate constants are available or unknown 5 @UST ChE Department 5 @UST ChE Department @UST ChE Department 6 1 Levenspiel Plot Graphical Method Yi = 1 −rAi X Ai = C A 0 − C Ai CA0 By Levenspiel ▪ Volume of a CSTR is represented partly by the area of rectangles bounded by the plot of 1/-rJ vs XJ, with height equal to 1/-rJm and width as ΔXJm Yi ▪ Volume of a PFR is represented partly by the area under the curve bounded by a plot of 1/-rJ vs XJ between 0 and XJ. XAi 7 8 @UST ChE Department 7 @UST ChE Department 8 Solution Methods Walas Plot ▪ Graphical ▪ ▪ Applicable if data of –rJ and CJ or XJ are available Uses: ▪ Levenspiel plots - Plot of 1/-rJ vs CJ or XJ ▪ or Walas Plot - Plot of -rJ vs CJ -rAi ▪ Numerical or Algebraic Methods ▪ Applicable if the kinetics of the reaction is known or unknown, meaning the order, rate constants are available or unknown CAi 9 @UST ChE Department 9 10 @UST ChE Department 10 Graphical Method Graphical Method ▪ Given the following –rA and CA data: By Walas (For CSTR only) CAi ▪ A plot of -rJ vs CJ is used -rAi CAo = 0.5 ▪ Each CSTR is represented by a straight line with an x-intercept of CJm-1 and slope of -1/τm ▪ For multiple CSTRs of same size, the slopes of each line must be the same. vo = 25 FAo = vo CAo = 12.5 vo = volumetric flow rate FAo = molar flow rate 11 @UST ChE Department 11 @UST ChE Department @UST ChE Department 12 2 Problems: Solution: Problem 2 2. Determine the volume of a PFR to achieve same conversion in (1) 1. Find the volume of a single CSTR needed to achieve 80% conversion of A. 2. Determine the volume of a PFR to achieve the same conversion in (1). 3. Suppose 2 equal sized CSTRs are connected in series. What total volume is needed for the same conversion? 4. Suppose equal sized CSTR and PFR are connected in series. What total volume is needed for the same conversion if CSTR is ahead? 5. Repeat 1-4 if the total volume is to be 60 ft3 and the final conversion is unknown. VPFR = For PFR: VPFR = vo FA FAo dFA rA XA = XA −CA0 dX A rA 0 −CA0 dX A rA 0 XA VPFR = FA0 0 VPFR = FA0 0.8 0 1 dX A −rA 1 dX A −rA VPFR = FAo*(Area under the curve) 13 14 @UST ChE Department 13 @UST ChE Department 14 Solution: Problem 2 (A PFR – Numerical Sol’n) Solution: Problem 2 (A PFR Graphical) 2. Determine the volume of a PFR to achieve same conversion in (1) 2. Determine the volume of a PFR to achieve same conversion in (1) Or: Using Simpson’s Rule with x = 0.40: Y1 = 2.6 at XA1 = 0.55 From Levenspiel Plot: 𝑥𝑛 Y2 = 7.5 at XA2 = 0.8-0.55 = 0.25 න XA = 0.8 and FAo = 12.5 lb-mole/hr VPFR = FA0 0.8 0 𝑓 𝑥 𝑑𝑥 ≈ 𝑥0 ℎ [𝑓 𝑥0 + 4𝑓 𝑥1 + 2𝑓 𝑥2 + 4𝑓 𝑥3 +. . . +4𝑓 𝑥𝑛−1 + 𝑓 𝑥𝑛 ] 3 XA = 0.8 and FAo = 12.5 lb-mole/hr 1 dX A −rA VPFR = FA0 0.8 0 x 1 dX A = FA0 A 3 −rA 1 −rA VPFR = FAo*(Area under the curve) XA=0 Integral=(2.6x0.55) + (7.5x0.25)] = 3.305 VPFR = (12.5) VPFR = 12.5(3.25) = 41.31ft 3 1 −rA +4 1 +2 2 XA=0.2 1 −rA 1 −rA +4 3 XA=0.4 XA=0.6 5 XA=0.8 0.2 1.176 + 4 (1.887 ) + 2 ( 3.226 ) + 4 (5.556 ) + 12.346 3 VPFR = 41.45 ft 3 15 16 @UST ChE Department 15 1 −rA + 4 @UST ChE Department 16 Solution: follow-up (2 PFRs instead of a PFR) Solution: follow-up (2 PFRs instead of a PFR) What if 2 Plug Flow Reactors were used instead of only one PFR, calculate the reactor volumes V1 and V2 for when the intermediate conversion is 40% and the final conversion is 80%. The entering molar flow rate is the same as in the previous examples, 12.5 lb-mole/hr. VPFR = FA0 0.4 0 1 dX A + −rA 0.8 0.4 What if 2 Plug Flow Reactors were used instead of only one PFR, calculate the reactor volumes V1 and V2 for when the intermediate conversion is 40% and the final conversion is 80%. The entering molar flow rate is the same as in the previous examples, 12.5 lb-mole/hr. 1 dX A −rA VPFR = FA0 For the first PFR: Using Simpson’s Rule with x = 0.2: VPFR1 = FA0 0.4 0 VPFR1 = (12.5) 1 dX A −rA = FA0 xA 3 1 −rA +4 1 1 −rA + 2 1 −rA @UST ChE Department 1 dX A + −rA 0.8 0.4 1 dX A −rA For the 2nd PFR: Using Simpson’s Rule with x = 0.2: VPFR2 = FA0 0.8 0.4 1 dX A = F x A A0 −rA 3 3 XA=0 XA=0.2 XA=0.4 0.2 (1.176 ) + 4 (1.887 ) + (3.226 ) VPFR1 = 9.96 ft 3 17 3 @UST ChE Department 17 0.4 0 VPFR2 = 12.5 0.2 3 1 −rA +4 1 1 −rA + 2 1 −rA 3 XA=0 XA=0.2 XA=0.4 ( 3.226 ) + 4 ( 5.556 ) + (12.346 ) VPFR2 = 31.49 ft 3 VT = 9.96 + 31.49 = 41.45 ft 3 18 @UST ChE Department 18 3 Solution: follow-up (2 PFRs instead of a PFR) Solution: Problem 3 3. Suppose 2 equal sized CSTRs are connected in series. What total volume is needed for the same conversion? What if 2 Plug Flow Reactors were used instead of only one PFR, calculate the reactor volumes V1 and V2 for when the intermediate conversion is 40% and the final conversion is 80%. The entering molar flow rate is the same as in the previous examples, 12.5 lb-mole/hr. A PFR: VPFR = FA0 0.4 1 dX A = FA0 −rA 0.4 0 1 dX A + −rA 0.8 0.4 xA1 xAo =0 2 PFRs in Series: 0.8 FA1 FAo 1 dX A −rA V1 FA2 xA2 = 0.80 V2 Therefore, Volume of a = Total volume of single PFR PFRs in series VT V1 V2 19 @UST ChE Department 19 @UST ChE Department 20 For CSTR: Solution by Walas Plot For single CSTR: V = Analysis of Continuous Reactors FAo -FA - rA For CSTRs in series: Vm = Lecture No. 6.1 B Graphical Method Walas Plot Fjm −1-Fjm Vm = vo Linearizing: - rjm = − 1 m Y Ch.E. 2115 Lola Domnina B. Pestaňo, Ph.D. Y = -rjm X = Cjm = - rjm C jm + m = v o (C jm −1-C jm ) - rjm C jm −1-C jm C jm −1 - rjm Eqn. of straight line m x m X-intercept =Cjm-1 b m=− 1 m=− m vo Vm 22 @UST ChE Department 21 22 Demonstration of Walas Method = m -rAi Problem Set 3F: (Use Levenspiel Plot) C jo - C j = 1. Suppose a PFR followed by CSTR are used whose intermediate conversion is 0.6. What total volume is needed for the same conversion (XA=0.8)? - rj C jm −1 - C jm - r jm 2. Suppose a PFR followed by CSTR of equal size are used. What total volume is needed for the same conversion(XA=0.8)? -rA1 m=− 1 1 CAi @UST ChE Department 3. Suppose 2 PFR of equal size are connected in series. What total volume is needed for the same conversion(XA=0.8)? CA0 CA1 23 PS 3F Graph ical 23 @UST Ch.E. Department 24 4 Problem Set 3F: (Use Levenspiel & Walas Plot) PS 3F Graph ical End. 4. Suppose 3 equal sized CSTRs are used. What total volume is needed for the same conversion (XA=0.8)? Use 2 Methods 5. Suppose 2 CSTRs of volumes 45ft3 and 20ft3 respectively are connected in series. What % conversion is expected? Use 2 Methods 26 @UST ChE Department 25 @UST ChE Department 26 5