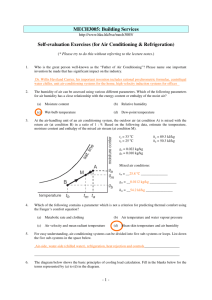
HVAC Thermodynamics Basics: Introduction to Engineering Principles
advertisement

1 Introduction Objectives After reading this chapter the student should be able to: • Refresh his knowledge on the engineering basics • Understand the laws of thermodynamics 1.1 General Air conditioning for human comfort was considered a luxury a few decades ago, but now it has become a necessity in life. The air conditioning industry is rapidly developing throughout the world. More than 10 million window installations are being installed each year and residential central cooling installations are enjoying similar popularity. Apart from reasons for comfort alone, air conditioning is commonly used nowadays in various industries such as food, automobiles, hotels, textiles and many more. On Earth, not only pollution from smoke is on the rise but pollution from dust is also playing havoc with our lives. Air conditioning plays a vital role in keeping out smoke and dust which could harm our health. Similarly, air conditioning has an important role to play in the preservation of food. At present, there is hardly any sector of the economy that is not dependent on this industry. In fact in most areas of industry, HVAC systems are considered to be a basic necessity. It is thus important to become part of this industry and this course is targeted at providing you with the basic knowledge and technology to play a role in designing, installing and commissioning HVAC systems. The following gives an overview of the basic principles of thermodynamics, which play a key role in understanding HVAC systems. 1.2 Principles of Thermodynamics 1.2.1 Force, Newtons In simple language, force is defined as a push or a pull. It is anything that has a tendency to set a body into motion, to bring a body to rest or change the direction of any motion. 2 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians 1.2.2 Pressure, Pascals Pressure is the force exerted per unit area. It may be described as the measure of intensity of a force exerted on any given point on the contact surface. Whenever a force is evenly distributed over a given area the pressure at any point on the surface is the same. It can be calculated by dividing the total force exerted by the area (on which the force is exerted). Atmospheric pressure The Earth is surrounded by an envelope of air called the atmosphere, which extends upward from the surface of the earth. Air has mass and due to gravity exerts a force called weight. The force per unit area is called pressure. This pressure exerted on the Earth’s surface is known as atmospheric pressure. Gauge pressure Most pressure measuring instruments measure the difference between the pressure of a fluid and the atmospheric pressure. This is referred to as gauge pressure. Absolute pressure Absolute pressure is the sum of gauge pressure and atmospheric pressure. Vacuum If the pressure is lower than the atmospheric pressure, its gauge pressure is negative and the term vacuum is applied to the magnitude of the gauge pressure when the absolute pressure is zero (i.e. there is no air present whatsoever). The relationships among absolute pressure, gauge pressure, atmospheric pressure and vacuum are shown graphically in the Figure 1.1. Figure 1.1 Relationship between absolute, gauge and vacuum pressures In the above figure Pa is the atmospheric pressure Pgauge is the gauge pressure Pab is the absolute pressure Pvacuum is the vacuum pressure Introduction 3 1.2.3 Density It is defined as the mass of a substance divided by its volume or the mass per unit volume. ρ = mass/volume Specific Volume is defined as the reciprocal of density or volume per unit mass. v = V/m Specific Weight (Ws) is defined as the weight of a substance divided by its volume or the weight per unit volume. Ws = m/V 1.2.4 Work If a system undergoes a displacement under the action of a force, work is said to be done; the amount of work being equal to the product of force and the component of displacement parallel to the force. If a system as a whole exerts a force on its surrounding and a displacement takes place, the work that is done either by or on the system is said to be external work. 1.2.5 Energy A body is said to possess energy when it is capable of doing work. In more general terms, energy is the capacity of a body for producing an effect. Energy is classified as 1. Stored Energy; examples are (a) Chemical energy in fuel and (b) Energy stored in dams 2. Energy in Transition: examples are (a) Heat and (b) Work The following are the various forms of energy. Potential energy (P.E) It is the energy stored in the system due to its position in the gravitational force field. If a heavy object such as a building stone is lifted from the ground to the roof, the energy required to lift the stone is stored in it as potential energy. This stored potential energy remains unchanged as long as the stone remains in its position. P×E = mgH Where H = height of the object above the datum ⎛ m⎞ Units ⎜ kg 2 ⎟m = N.m = Joules ⎝ s ⎠ Kinetic Energy (K.E), Joules= Newton meter If a body weighing one kg is moving with a velocity of v m/sec with respect to the observer, then the kinetic energy stored in the body is given by: K.E = 1 mv 2 2 This energy will remain stored in the body as long as it continues in motion at a constant velocity. When the velocity is zero, the kinetic energy is also zero. Internal Energy Molecules possess mass. They possess motion of transactional and rotational nature in liquid and gaseous states. Owing to the mass and motion these molecules have a large amount of kinetic energy 4 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians stored in them. Any change in the temperature results in the change in the molecular kinetic energy since molecular velocity is a function of temperature. Also the molecules are attracted towards each other by forces, which are very large in their solid state and tend to vanish once they are in a perfect gas state. In the melting of a solid or vaporization of a liquid it is necessary to overcome these forces. The energy required to bring about this change is stored in molecules as potential energy. The internal energy is defined as the total energy of the body - chemical, nuclear, heat, gravitational, or any other type of energy. This energy is stored within the body which is denoted by the symbol ‘µ’. It is obvious from the above definition that it is impossible to measure the absolute value of the internal energy. However, we can measure the changes occurring in the internal energy. Since thermodynamics deals with the change in the internal energy of the system, it is important to know what causes the internal energy to change. The change in the internal energy can be caused by either due to absorption or release of heat in the system or the work done by or on the system., or if any matter enters or leaves the system. 1.2.6 Heat Heat is one of the many forms of energy. This is evident from the fact that heat can be converted into other forms of energy and that other forms of energy can be converted into heat. Heat as molecular energy is universally accepted and heat as internal energy of the matter is thermodynamics. Since all other forms of energy may be converted into heat, it is considered to be energy in its lowest form. The availability of heat energy to do work depends on temperature differential. 1.2.7 Heat capacity It may be defined as the energy that must be added or removed from one kilogram of a substance to change its temperature by one degree Centigrade. In refrigeration technology heat capacity is used to determine how much heat should be removed to refrigerate various products. 1.2.7.1 Sensible heat (QS) Heat which results in an increase or decrease in the temperature without it changing its phase is called sensible heat. A change in sensible heat is given by the equation when there is a change in temperature QS = m× CS (T2 – T1) Note: CS is the heat capacity at constant pressure m = mass of the substance in kg (T2 – T1) = Temperature difference in °C 1.2.7.2 Latent heat (QL) Latent heat is the heat at which a substance changes its phase without any increase or decrease in the temperature. It is the amount of heat required to change the state of a substance. QL = m×Cw(w2 – w1) Note: Cw is the heat capacity of moisture m = mass of the substance in kg (w2 – w1) = change in moisture content in g/kg Introduction 5 1.2.7.3 Total heat (QT) Total heat is the sum of sensible heat and latent heat. Heat measurements are taken above a specified datum. These measurements with water are at zero degrees C, since below this temperature water is solid. Refrigerant heat measurements are at –400C. For example: The sensible heat, latent heat and total heat for steam are shown in Figure 1.2 below. QT = QS + QL Figure 1. 2 Total Heat Chart Of –400C Ice To Steam at 100 0C a-b is sensible heat, b-c is latent heat of fusion, c-d is Sensible heat, d-e is latent heat of vaporization, ef is super heat. 1.3 Temperature and its measurement Temperature is a property of matter. It is the measure of intensity of heat contained in matter and its relative value. A substance is said to be hot or cold when its temperature is compared with some other reference temperature. A high temperature indicates a high level of heat intensity or thermal pressure and a body is said to be hot. Like other forms of energy heat can be measured because it has quantity and intensity. Heat is not visible but manifests itself in its effects on various substances either by changing its state or by creating relative degrees of sensation when in contact with the human body. Since temperature is a measure of heat content, the temperature can be measured by measuring the effects of heat on different properties of matter as follows; • Addition of heat increases the volume of the substance or pressure at constant volume. This property is used for measuring the temperature with the help of a mercury thermometer. • With the increase in temperature, the resistivity of metals increases which is utilized in resistance thermometers • If two junctions made of two dissimilar metals are maintained at different temperatures, a current flows in the circuit. This property is used in measuring with a thermocouple. 6 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians • When the temperature of a substance increases, the color also changes. This property is used for measuring the temperature in radiation pyrometers. 1.4 Pressure and temperature relationship Water boils at 1000C when the pressure on it is atmospheric at sea level. If the pressure is increased above the atmospheric pressure, i.e. in a deep mine shaft the boiling point increases and when the pressure is reduced below atmospheric, i.e. on top of a mountain, it reduces. Boiling water does not necessarily have to be hot because if there is vacuum, water boils at a very low temperature. The same is true when it comes to other liquids, such as various refrigerants. These refrigerants have the same properties as water except their boiling point ranges are lower. This pressure temperature relationship is used in most air conditioning and refrigeration systems. 1.5 Laws of Thermodynamics 1.5.1 First law of Thermodynamics and Energy Conservation It is a fundamental principle that matter can neither be created nor destroyed though it may be made to take different forms. Similarly, energy cannot be created or destroyed. It can be converted from one form to another. The first law of thermodynamics states that the total energy in a system always remains constant. This law is mainly based on observation and can be best studied with the help of observations. In the following examples, we can see that heat, work, electricity and chemical energy are various forms of energy and they are mutually convertible. • • • • An electric Iron converts electricity into heat. An electric fan converts electricity into work. Water flowing through a turbine converts its potential energy into work. Churning of water converts work into heat. The first law of thermodynamics can be represented by the equation: E1 + Qa – Qt = E2 Where: E1 is the energy possessed by the system initially E2 is the energy possessed by the system after the work is done Qa is the energy added to the system Qt is the energy taken away from the system. 1.5.2 Second law of Thermodynamics The second law of thermodynamics can be stated in a number of ways as: • Heat flows from a body at higher temperature to a body at lower temperature irrespective of the mass and material of the body participating in the heat transfer. This heat flow is possible without the addition of external work. • Work has the tendency to convert into heat but the heat cannot be converted into work. • Every engine or a refrigerator ejects heat to the surroundings. Introduction 7 With a brief discussion on the various thermodynamic principles, let us now study the fundamentals of Heating, Ventilation and Air conditioning in the next chapters. 1.6 Fundamentals of Heat Transfer 1.6.1 Modes of Transferring Heat Heat is always transferred when a temperature difference exists between two bodies. There are three basic modes of heat transfer: • Conduction involves the transfer of heat by the interactions of atoms or molecules of a material through which the heat is being transferred. • Convection involves the transfer of heat by the mixing and motion of macroscopic portions of a fluid. • Radiation, or radiant heat transfer, involves the transfer of heat by electromagnetic radiation that arises due to the temperature of a body. 1.6.2 Heat Flux The rate at which heat is transferred is represented by the symbol. Common units for heat Q transfer rate is Watts. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol. Units for heat flux are W/m2. The heat flux can be Qhf determined by dividing the heat transfer rate by the area through which the heat is being transferred. Where: 1.6.3 Q Qhf = -------A Qhf = heat flux (W/m2) Q = heat transfer rate (W) A = area (m2) Thermal Conductivity The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity (k) measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a solid by conduction. The thermal conductivity of most liquids and solids varies with temperature. For vapors, it depends upon pressure. 1 W/(m.K) = 1 W/(m.oC) = 0.85984 kcal/(hr.m.oC) 8 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians Table: 1.1 Thermal conductivity values for various materials at 300 K Material Copper Gold Aluminum Iron Carbon steel Stainless Steel (18/8) Glass Plastics Wood (shredded/cemented) Cork Water Ethylene glycol Hydrogen Benzene Air 1.6.4 Thermal conductivity W/m.K 399 317 237 80.2 43 15.1 0.81 0.2 – 0.3 0.087 0.039 0.6 0.26 0.18 0.159 0.026 Log Mean Temperature Difference (LMTD) In heat exchanger applications, the inlet and outlet temperatures are commonly specified based on the fluid in the tubes. The temperature change that takes place across the heat exchanger from the entrance to the exit is not linear. A precise temperature change between two fluids across the heat exchanger is best represented by the log mean temperature difference (LMTD or ΔTlm). (ΔT2 - ΔT1) Δ TLM = ---------------------------In (ΔT2 / ΔT1) Where: ΔT2 = the larger temperature difference between the two fluid streams at either the entrance or the exit to the heat exchanger ΔT1 = the smaller temperature difference between the two fluid streams at either the entrance or the exit to the heat exchanger 1.6.5 Convective Heat Transfer Coefficient The convective heat transfer coefficient (hc), defines, in part, the heat transfer due to convection. The convective heat transfer coefficient is sometimes referred to as a film coefficient and represents the thermal resistance of a relatively stagnant layer of fluid between a heat transfer surface and the fluid medium. Common units used to measure the convective heat transfer coefficient are (W/m2K). Introduction 9 Table 1.2 Typical order-of magnitude values of convective heat transfer coefficients Type of fluid and flow Convective heat transfer coefficient 2 hc (W/m K) , Air, free convection Water, free convection Air or superheated steam, forced convection Oil, forced convection Water, forced convection Synthetic refrigerants, boiling Water, boiling Synthetic refrigerants, condensing Steam, condensing 1.6.7 6 – 30 20 – 100 30 – 300 60 – 1800 300 – 18000 500 - 3000 3000 – 60000 1500 - 5000 6000 – 120000 Overall Heat Transfer Coefficient In the case of combined heat transfer, it is common practice to relate the total rate of heat transfer Q the overall cross-sectional area for heat transfer (Ao), and the overall temperature difference (ΔTo) using the overall heat transfer coefficient (Uo). The overall heat transfer coefficient combines the heat transfer coefficient of the two heat exchanger fluids and the thermal conductivity of the heat exchanger tubes. Uo is specific to the heat exchanger and the fluids that are used in the heat exchanger. Q = Uo Ao ΔTo Where: 1.6.8 Q = The rate of heat transfer (W) Uo = the overall heat transfer coefficient (W/m2 oK) Ao = the overall cross-sectional area for heat transfer (m2) ΔTo = the overall temperature difference (oK) Bulk Temperature The fluid temperature (Tb), referred to as the bulk temperature, varies according to the details of the situation. For flow adjacent to a hot or cold surface, Tb is the temperature of the fluid that is "far" from the surface, for instance, the center of the flow channel. For boiling or condensation, Tb is equal to the saturation temperature. 1.7 Fundamentals of Fluid Flow Fluid flow is an important part of most industrial processes; especially those involving the transfer of heat. Frequently, when it is desired to remove heat from the point at which it is generated, some type of fluid is involved in the heat transfer process. Examples of this are the cooling water circulated through cooling coils in HVAC, the air flow past the heating and cooling coils, from fans and blowers, duct work, terminal units, packaged air conditioning units etc., Unlike solids, the particles of fluids move through piping and components at different velocities and are often subjected to different accelerations. The basic principles of fluid flow include three concepts or principles: (1) The first is the principle of momentum (Equations of fluid forces) (2) The second is the conservation of energy (First Law of Thermodynamics). (3) The third is the conservation of mass (Continuity equation) 10 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians 1.7.1 Properties of Fluids A fluid is any substance which flows because its particles are not rigidly attached to one another. This includes liquids, gases and even some materials which are normally considered solids, such as glass. Fluids are materials which have no repeating crystalline structure. There are several properties, including temperature, pressure, mass, specific volume, density, and Buoyancy. • Temperature was defined as the relative measure of how hot or cold a material is. It can be used to predict the direction that heat will be transferred. • Pressure was defined as the force per unit area. Common units for pressure are Pascal. • Mass was defined as the quantity of matter contained in a body and is to be distinguished from weight, which is measured by the pull of gravity on a body. • The specific volume of a substance is the volume per unit mass of the are substance. Typical units are m3/kg . • Density, on the other hand, is the mass of a substance per unit volume. Typical units are kg/m3. Density and specific volume are the inverse of one another. Both density and specific volume is dependant on the temperature and somewhat on the pressure of the fluid. As the temperature of the fluid increases, the density decreases and the specific volume increases. Since liquids are considered incompressible, an increase in pressure will result in no change in density or specific volume of the liquid. In actuality, liquids can be slightly compressed at high pressures, resulting in a slight increase in density and a slight decrease in specific volume of the liquid. • Buoyancy is defined as the tendency of a body to float or rise when submerged in a fluid. When a body is placed in a fluid, it is buoyed up by a force equal to the weight of the water that it displaces. • Compressibility is the measure of the change in volume a substance undergoes when a pressure is exerted on the substance. Liquids are generally considered to be incompressible. For instance, a pressure of 1110 kg/ cm 2 will cause a given volume of water to decrease by only 5% from its volume at atmospheric pressure. Gases on the other hand, are very compressible. The volume of a gas can be readily changed by exerting an external pressure on the gas. 1.7.2 Pascal’s Law Pascal's law, or the Principle of transmission of fluid-pressure, states that "pressure exerted anywhere in a confined incompressible fluid is transmitted equally in all directions throughout the fluid such that the pressure ratio (initial difference) remains the same." where ΔP is the hydrostatic pressure (given in pascals in the SI system), or the difference in pressure at two points within a fluid column, due to the weight of the fluid; ρ is the fluid density (in kilograms per cubic meter in the SI system); g is acceleration due to gravity (normally using the sea level acceleration due to Earth’s gravity in metres per second squared); Δh is the height of fluid above the point of measurement, or the difference in elevation between the two points within the fluid column (in metres in SI). Introduction 11 1.7.3 Control Volume In thermodynamics, a control volume was defined as a fixed region in space where one studies the masses and energies crossing the boundaries of the region. This concept of a control volume is also very useful in analyzing fluid flow problems. The boundary of a control volume for fluid flow is usually taken as the physical boundary of the part through which the flow is occurring. The control volume concept is used in fluid dynamics applications, utilizing the continuity, momentum, and energy principles 1.7.4 Volumetric Flow Rate The volumetric flow rate V of a system is a measure of the volume of fluid passing a point in the system per unit time. The volumetric flow rate can be calculated as the product of the cross sectional area (A) for flow and the average flow velocity (v). ˙V = A x v The area is measured in square meter and velocity in meters per second, results in volumetric flow rate measured in cubic meter per second. Other common units for volumetric flow is liters per minute. 1.7.5 Mass Flow Rate The mass flow rate (m ) of a system is a measure of the mass of fluid passing a point in the system per unit time. The mass flow rate is related to the volumetric flow rate. Mass flowrate = Density x Volumetric flowrate m=ρxV The volumetric flow rate is in m 3 /s and the density is kg/m 3 results in mass flow rate measured in kilograms per second 1.7.8 Conservation of Mass In thermodynamics, we know that the energy can neither be created nor destroyed, only changed from one form to another form. The same is true for mass. Conservation of mass is a principle of engineering that states that all mass flow rates into a control volume are equal to all mass flow rates out of the control volume plus the rate of change of mass within the control volume. Δm mIN = mOUT + ------Δt 1.7.9 Steady-State Flow Steady-state flow refers to the condition where the fluid properties at any single point in the system do not change over time. These fluid properties include temperature, pressure, and velocity. One of the most significant properties that is constant in a steady-state flow system is the system mass flow rate. This means that there is no accumulation of mass within any component in the system. 12 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians 1.7.10 Continuity Equation The continuity equation is simply a mathematical expression of the principle of conservation of mass. For a control volume that has a single inlet and a single outlet, the principle of conservation of mass states that, for steady-state flow, the mass flow rate into the volume must equal the mass flow rate out. The continuity equation for this situation is expressed by the following equation: mIN = mOUT ρ x A x v (inlet) = ρ x A x v (Outlet) 1.7.11 Head Loss Head loss is a measure of the reduction in the total head (sum of elevation head, velocity head and pressure head) of the fluid as it moves through a fluid system. Head loss is unavoidable in real fluids. It is present because of: the friction between the fluid and the walls of the pipe; the friction between adjacent fluid particles as they move relative to one another; and the turbulence caused whenever the flow is redirected or affected in any way by such components as piping entrances and exits, pumps, valves, flow reducers, and fittings. 1.7.12 Frictional Loss Frictional loss is that part of the total head loss that occurs as the fluid flows through straight pipes. The head loss for fluid flow is directly proportional to the length of pipe, the square of the fluid velocity, and a term accounting for fluid friction called the friction factor. The head loss is inversely proportional to the diameter of the pipe. Lv2 Heat loss ∝ f ------------D 1.7.13 Frictional Factor The friction factor has been determined to depend on the Reynolds number for the flow and the degree of roughness of the pipe’s inner surface. The quantity used to measure the roughness of the pipe is called the relative roughness, which equals the average height of surface irregularities “∈”divided by the pipe diameter “D” ∈ Relative Roughness = ---------------D The value of the friction factor is usually obtained from the Moody Chart. Introduction 13 1.7.14 Darcy’s Equation The frictional head loss can be calculated using a mathematical relationship that is known as Darcy’s equation for head loss. The equation takes two distinct forms. The first form of Darcy’s equation determines the losses in the system associated with the length of the pipe. L v2 Hf = f ----- x -----D 2g Where: 1.7.15 f = friction factor (unitless) L = length of pipe (meters) D = diameter of pipe (meters) v = fluid velocity (m/sec) g = gravitational acceleration (m/sec2) Minor Losses The losses that occur in pipelines due to bends, elbows, joints, valves, etc. are sometimes called minor losses. This is a misnomer because in many cases these losses are more important than the losses due to pipe friction, considered in the preceding section. For all minor losses in turbulent flow, the head loss varies as the square of the velocity. Thus a convenient method of expressing the minor losses in flow is by means of a loss coefficient (k). Values of the loss coefficient (k) for typical situations and fittings is found in standard handbooks. The form of Darcy’s equation used to calculate minor losses of individual fluid system components is expressed by Equation: v2 Hf 1.7.16 = k -----------2g Equivalent Piping Length Minor losses may be expressed in terms of the equivalent length (Leq) of pipe that would have the same head loss for the same discharge flow rate. This relationship can be found by setting the two forms of Darcy’s equation equal to each other. L v2 Hf = f ----- x -----D 2g v2 = k ------------2g This yields two relationships that are useful. D Leq Leq = k --------- and k = f -------------f D 14 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians 2 Psychrometry Objectives At the conclusion of this chapter, students should be able to: • Understand psychrometry and read a psychrometric chart. • Construct a psychrometric chart • Describe various psychrometric processes • Understand various air-conditioning systems 2.1 Introduction to psychrometry Psychrometry is a science that involves the property of moist air (a mixture of dry air and water vapor) and the process in which the temperature and/or the water vapor content of the mixture are changed. As per ASHRAE definition, the psychrometry as that branch of physics concerned with the measurement or determination of atmospheric conditions, particularly the moisture in the air. The Psychrometric chart is a convenient tool for determining the moist air psychrometric properties and visualizing the changes of moist air properties in various sequences of psychrometric processes. These charts are also drawn on the basis of specified barometric pressure or elevation with respect to the sea level. The Psychrometric tables exhibit more accurate changes occurring in air and moisture mixtures in the air conditioning processes, but the psychrometric charts are more convenient to use in all practical purposes. 2.2 The properties of air The atmospheric air is a mixture of dry air and water vapor (moisture). The air in natural state; always contain certain amount (3.5%) of water vapor. The dry air and water vapor, do not react chemically with one another. Although they are present as mixture, each acts independent of the other. 2.2.1 Dalton’s law Dalton’s law states that two gases can occupy the same space (Volume) at the same time, but each acts independently of the other and each exerts its own pressure. 16 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians Total pressure = Partial pressure of dry air + partial pressure of water vapor. In common usage, total pressure is referred to as “Barometric Pressure” or “Atmospheric Pressure”. 2.2.2 Air density and specific volume Air has its own weight. The density of standard air is 1.2 kg/m3 and specific volume 0.83 m3/kg For example, a fan in an air conditioning system is 300 m3/min, Then the weight of the air handled will be 300 x 1.2 = 360 kg/min. 2.2.3 Dry air The dry air in the atmosphere is mixture of oxygen (21%) and nitrogen (78%). The balance (1%) consists of other gases, such as argon, carbon dioxide, hydrogen etc. Both oxygen and nitrogen are in highly superheated state and therefore, the dry air is also in super heated state. Due to this state, the air conditioning processes make only slight changes in the density/ volume of dry air. When dry air is heated or cooled, only the sensible heat is added or deleted, without any effect on the latent heat. The specific heat of dry air = 0.133 kcal/kg 2.2.4 Moist air It is a mixture of dry air and water vapor. The content of water vapor depends upon the temperature of air and its quantity may change from zero to maximum, i.e the saturation capacity of air. The mass of water vapor associated with the dry air is not constant. But how the water vapor is added to the dry air? The following points will illustrate how this is being carried out: (a) The water vapor constantly evaporating from the lake, sea and oceans into the earth’s atmosphere and returns as precipitation to the earth. (b) Water vapor is added to the air from our homes, buildings by infiltration, perspiration, respiration, cooking, cloth washing, plants and trees from residential areas and forest. (c) Water vapor is added to the air from the building materials and furnishings (d) Water vapor is added to the air by humidification or evaporative cooling processes. The table below shows the composition of the water vapor for calculating the molecular mass. Table 2.1 Composition of water vapor Substance Hydrogen (H2) Oxygen (O) Total Atoms 2 1 Atomic mass 1.00794 15.9994 Molecular mass 2.01588 15.99940 18.01528 The pressure exerted by the water vapor in a mixture of air, will depend upon the amount of vapor present or the percentage of saturation. It is a known fact that the saturation pressure will be achieved only if the water and vapor formed are inside a container. Therefore, it is obvious that the pressure of water vapor present in atmosphere need not be the saturation pressure at the corresponding temperature. Psychrometry 17 The density of water vapor is very low and it is 0.0253 kg/m3. So the smaller units of grams (g) or grains (gr) are used to express its density. (1 Lb = 7000 grains) (1 grain=0.06g). The following table shows the saturated water vapor and density at different temperatures. Table 2.2 Saturated vapor pressure 2.2.5 Dry Bulb Temperature, t db Dry-bulb temperature is the temperature of the air measured by an ordinary thermometer or a temperature sensor like thermocouple, thermister, RTD, bi-metal and mercury bulb It is the true temperature of moist air at rest, and not subjected to evaporation, condensation or radiation. Since air is a mixture of dry air and water vapor, the dry-bulb temperature is the temperature of not only the dry air component but also the temperature of the water –vapor component. 18 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians The usage of dry-bulb temperature measurement; (a) In calculating the sensible energy knowing the beginning and ending points, the mass flow of air, and the specific heat capacity of the moist air. q = m Cp (t2 – t1) Where: (b) (c) 2.2.6 q = Sensible heat m = Mass of dry-air) Cp = Specific heat of water vapor t2 = Entry temperature t1 = Exit temperature In psychrometric charts as bottom X-axis coordinate, to calculate other properties of moist air To calculate Enthalpy of mixed air (dry air + Water vapor) at a particular temperature measured. Wet Bulb Temperature, twb The temperature measured by the thermometer with its bulb covered with a wet cloth and exposed to a current of moving air at 3 to 4 m/s is known as wet bulb temperature (WBT). As the air passes over the wet wick of the thermometer the water of the wick tends to evaporate. The cooling effect of evaporation lowers the temperature measured by the wet bulb thermometer corresponding to the rate of evaporation. When the temperature measured by the WBT reaches a steady state, then the heat absorbed by the bulb for evaporation of water vapor is equal to the heat given by air (by convection) to the thermometer. This means that the total heat of air leaving the thermometer remains constant. The heat necessary to cause evaporation in the manner stated above is present in air in the form of sensible heat. During the process of evaporation, sensible heat is converted into latent heat of vaporization maintaining the total heat of air constant. This conversion to latent heat is accomplished without change in total heat. The evaporation rate from the wet wick depends on the condition of the air passing over it. If the surrounding air is dry then the evaporation rate will be more rapid and the drop in temperature (difference between temp. measured by WBT and DBT) will be appreciable. When the surrounding air is moist, then the evaporation rate will be slower; so will be the drop in temperature. This shows that the wet bulb temperature is a measure of degree of saturation or the relative humidity. Air with high relative humidity will have lesser drop in temperature compared to air with low relative humidity. Air with 100% relative humidity will have no drop in temperature. The equipment used for measuring dry bulb temperature and wet bulb temperature simultaneously is called a psychrometer. There are different types of psychrometers, as listed below. (a) Laboratory Psychrometer This is a simple instrument, which houses both the dry bulb thermometer and the wet bulb thermometer. This is generally used in college laboratories.(Figure 2.1) (b) Sling Psychrometer This psychrometer consists of two mercury thermometers mounted on a frame, which has a handle. The handle of the frame helps in the rotating of the psychrometer to produce the necessary air motion. One bulb of the two thermometers is covered with a wet wick to measure the WBT. The Psychrometry 19 rotating motion of the sling provides necessary air velocity over the thermometers. This air movement passing the wick helps to bring the air at temperature (WBT) in immediate contact with the wet wick.(Figure 2.2) (c) Aspirating Psychrometer This is similar to the other psychrometers with the exception of the blower, which provides a rapid motion of air over the thermometers. These types are used to measure the temperatures after a particular period of time mostly to measure the atmospheric conditions of cities throughout the day and year. The motor is connected to the time switch as per the interval required for the measurement of temperature.(Figure 2.3) Figure 2.1 Laboratory Psychrometer Figure 2.2 Sling Psychrometer 20 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians Figure 2.3 Aspirating Psychrometer 2.2.7 Relative Humidity Relative Humidity can be defined in two ways: “The ratio of the actual amount of moisture content in one unit volume of dry air at a certain temperature to the amount of moisture needed to saturate it at that temperature” “The ratio of the actual pressure of water vapor of a certain unsaturated moist air at a given temperature to the vapor pressure when saturated at the same temperature”. Relative humidity signifies the absorption capacity of air. More moisture will be absorbed by air if the initial relative humidity is less. It is derived by the equation: PV φ = ----------------PVS Where Pv is vapor pressure; Pvs is saturated vapor pressure. Referring to the table on saturated vapor pressure shown; At 21.1ºC (70ºF), the air is holding 0.072 g/cc of moisture and that is saturated At 26.7 ºC (80ºF), the air is holding 0.098 g/cc of moisture and that is saturated For the above two conditions, the relative humidity is 100% Psychrometry 21 Here, if we need to increase the temperature only from 21.1ºC to 26.7ºF without increasing the moisture content, then the relative humidity will be: Table 2.3 Relationship between temperature, density and RH Temperature ºC (ºF) Actual Density g/cc 21.1 (70) 26.7 (80) 32.2 (90) 37.8 (100) 0.072 0.072 0.072 0.072 Saturated Density g/cc 0.072 0.098 0.133 0.177 Relative Humidity % 100% 73.4% 54.1% 40.7% The above chart indicate that if we increase the temperature of the air without increasing the moisture content, the relative humidity comes down and actually the air being dried. Drying is really means the removal or reduction of water vapor content As the temperature increases, the amount of water vapor needed for saturation also increases. When we push more water vapor into the same volume of air, the pressure exerted by the water vapor increases. This is seen from the table also. But increasing the temperature without adding water vapor, the pressure increase is appreciably low. 2.2.8 Dew point The temperature at which the water vapor contained in an air sample just starts to condense is called its “Dew Point”. Another defining statement is that the dew point is the temperature at which the moisture contained in the air at a particular temperature becomes saturated. When the R.H value reaches 100% with respect to the temperature and the moisture content, for example, at 21.1ºC, the moisture content is 0.072 g/cc, the relative humidity is 100% and any further cooling of air below 21.1ºC, some of the moisture will condense into water. Any object at a temperature below the dew point of the surrounding air, it will condense some moisture out of the air. the sweating observed on the outside of a glass of ice water is due to the condensation of moisture from air on to the cold surface of the glass. When we need to remove moisture from the air and condense it to liquid water, only the latent heat of the amount of water vapor to be condensed has also to be removed.. Since the latent heat of water being high,-an average of 555 kacl/kg of moisture-the load on the cooling system increases. 2.2.9 Humidity ratio Humidity ratio is the ratio of the mass of water vapor to the mass of dry air, in a sample or volume of moist air. mwv W = ----------mda Where; W = Humidity ratio in kgwv/kgda mwv = mass of water vapor in the space or sample of moist air mda = mass of dry air in the space or sample of moist air. The measurement of humidity ratio can be done by utilizing “Gravimetric Hygrometer” 22 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians The following equations are derived from the humidity ratio, water vapor pressure and relative humidity. The Ideal gas equation: pV = mRT Humidity ratio: W = mwv/mda Rearranging the ideal gas equation: m = pV/RT As “m” mass represents the mass of air, which is the sum of mwv & mda, we can individually write the equation as: Mass of water vapor mwv = pwv Vwv / Rwv Twv mda = pda Vda / Rda Tda As per Dalton’s Law, the water vapor and dry air occupy the same volume and are at the same temperature. Therefore eliminating the volume and temperatures terms: mwv = pwv / Rwv mda = pda / Rda Here again, Rwv and Rda represent the Specific Gas Constant for water vapor and dry air. Therefore, Rwv = R / M, Where, R = Molecular Gas Constant Mda= Molecular mass of dry air Mwv= Molecular mass of water vapor Therefore, Rda Rwv Therefore, = 8314.1 = 28.9645 = 18.01528 = 8314.1 / 28.9645 = 287.04 J/kg.K = 8314.1 / 18.0152 = 461.520 J/kg.K mwv pwv / Rwv W = ----------- = ------------------------ = mda pda / Rda pwv W = ----------- X pda Rda 287.04 x pwv ----------- = ---------------------------Rwv 461.52 x pda pwv W = 0.62198 x ---------------pda Total pressure, pbar = pwv + pda (or) pda = pbar – pwv 0.62198 x pwv The equation for humidity ratio can be written as W = ---------------------pbar - pwv Psychrometry 23 Since, RH equation, 100 x pwv = RH x pwvs, 0.62198 x RH x pwvs, / 100 Then W =-----------------------------------------pbar – RH x pwvs, / 100 Where: pwv pda pbar pwvs R.H = Partial pressure of the water-vgapor component of moist air mixture = Partial pressure of the dry air component of the moist air mixture = Total pressure, i.e., atmospheric or barometric pressure = Partial pressure of saturated water vapor at dry-bulb temperature = Relative humidity expressed in percentage Humidity ratio is sometimes incorrectly called “Specific Humidity” or “Absolute Humidity” To avoid this confusion, both specific humidity and absolute humidity is defined as follows: “Specific humidity is the weight of water vapor in unit mass of dry air (g/kg)” “Absolute humidity is the weight of moisture per unit volume of dry air (g/cc)” 2.2.10 Sensible heat flow Sensible heat is dry heat causing change in temperature but not in the moisture content. The sensible heat flow can be expressed as Qs = cp ρ q Δt / 3600 Where: 2.2.11 Qs = sensible heat flow (kW) cp = specific heat of air (kJ/kg K) = 1.0 kJ/kg.k ρ = air density at standard conditions = 1.202kg/m3 q = air flow (m3/hr) Δt = temperature (oC) Latent heat flow Latent heat is the heat, when supplied to or removed from air, results in a change of moisture content the temperature of the air is not changed The latent heat flow can be expressed as: Ql = hwe ρ q Δx / 3600 Where 2.2.12 Ql = latent heat flow (kW) hwe = 2465.56 - latent heat of vaporization of water (kJ/kg) ρ = 1.202 - air density at standard conditions (kg/m3) q = air flow (m3/hr) Δx = humidity ratio difference (kg water/kg dry air) Specific Volume The Specific volume in psychrometrics is the volume per unit mass of the dry air component and expressed as m3 / kgda The specific volume is used in process calculations in converting between moist air volumetric flow (m3/s) and the mass flow (kgda/s) of the dry air component. 24 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians The equation for specific volume , applying Ideal Gas Equation is as follows: Rda x T 287.055 J/ (kg.k) x (Tc + 273.15) v = ----------------- = -------------------------------------------- m3/kgda pbar - pwv (pbar - pwv) 2.2.13 Enthalpy or Heat content of air “The enthalpy of moist air is the sum of the enthalpy of the dry air and the enthalpy of the water vapour. In atmospheric air, water vapor content varies from 0 to 3% by mass. The enthalpy of moist air includes the: 1. Enthalpy of the dry air – The sensible heat 2. Enthalpy of water vapor – The latent heat For moist air, the enthalpy of dry air is given a zero value at 0°C, and for water vapour the enthalpy of saturated water is taken as zero at 0°C. The enthalpy of moist air is given by (h) h = ha + W hw Where: h = specific enthalpy of moist air (kJ/kg) ha = specific enthalpy of dry air (kJ/kg) W = humidity ratio ( kgwv / kgda) hw = specific enthalpy of water vapor (kJ/kg) Specific Enthalpy of Dry Air - Sensible Heat (ha) Assuming constant pressure conditions the specific enthalpy of dry air can be expressed as: ha = cpa t Where: cpa = specific heat capacity of air at constant pressure (kJ/kg°C) (For air temperature between -100°C and 100°C the specific heat capacity can be set to cpa = 1.006 (kJ/kg°C) t = air temperature (°C) Specific Enthalpy of Water Vapor - Latent Heat (hw) Assuming constant pressure conditions the specific enthalpy of water vapor can be expressed as: hw = cpw t + hwe Where cpw = specific heat of water vapor at constant pressure (kJ/kg°C) t = water vapor temperature (°C) hwe = evaporation heat of water at °C (kJ/kg) For water vapor the specific heat capacity can be set to cpw = 1.84 kJ/kg°C The heat of evaporation (water at °C) can be set to hwe = 2501 kJ/kg Psychrometry 25 Therefore, the enthalpy of moist air is summed up as: h = cpa t + W [cpw t + hwe] Where cpa= specific heat of dry air at constant pressure, kJ/kg°C, 1.006 kJ/kg°C cpw= specific heat of water vapor, kJ/kg°C, 1.84 kJ/Kg°C t = Dry-bulb temperature of air-vapor mixture, °C W = Humidity ratio, kg of water vapor/kg of dry air hwe = enthalpy of water vapor at temperature t, kJ/kg The unit of h is kJ/kg of dry air. Substituting the approximate values of cpa and cpw ,we obtain: h = 1.006 t + W (1.84 t + 2501) 2.3 Understanding the psychrometric charts 2.3.1 Dry Bulb Temperature – Tdb The Dry Bulb temperature, usually referred to as air temperature, is the air property that is most common used. When people refer to the temperature of the air, they are normally referring to its dry bulb temperature. Figure 2.4 Dry-bulb Temperature The Dry Bulb Temperature refers basically to the ambient air temperature. It is called "Dry Bulb" because the air temperature is indicated by a thermometer not affected by the moisture of the air. Dry-bulb temperature - Tdb, can be measured using a normal thermometer freely exposed to the air but shielded from radiation and moisture. The temperature is usually given in degrees Celsius (oC) or degrees Fahrenheit (oF). The SI unit is Kelvin (K). Zero Kelvin equals to - 273oC. The dry-bulb temperature is an indicator of heat content and is shown along the bottom axis of the psychrometric chart. Constant dry bulb temperatures appear as vertical lines in the psychrometric chart. 26 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians 2.3.2 Wet Bulb Temperature - Twb The Wet Bulb temperature is the temperature of adiabatic saturation. This is the temperature indicated by a moistened thermometer bulb exposed to the air flow. Wet Bulb temperature can be measured by using a thermometer with the bulb wrapped in wet muslin. The adiabatic evaporation of water from the thermometer and the cooling effect is indicated by a "wet bulb temperature" lower than the "dry bulb temperature" in the air. Figure 2.5 Wet-Bulb Temperature The rate of evaporation from the wet bandage on the bulb, and the temperature difference between the dry bulb and wet bulb, depends on the humidity of the air. The evaporation is reduced when the air contains more water vapor. The wet bulb temperature is always lower than the dry bulb temperature but will be identical with 100% relative humidity (the air is at the saturation line). Combining the dry bulb and wet bulb temperature in a psychrometric diagram or Mollier chart, gives the state of the humid air. Lines of constant wet bulb temperatures run diagonally from the upper left to the lower right in the Psychrometric Chart. 2.3.3 Dew Point Temperature - Tdp The Dew Point is the temperature at which water vapor starts to condense out of the air, the temperature at which air becomes completely saturated. Above this temperature the moisture will stay in the air. Psychrometry 27 Figure 2.6 Dew Point Temperature If the dew-point temperature is close to the air temperature, the relative humidity is high, and if the dew point is well below the air temperature, the relative humidity is low. If moisture condensates on a cold bottle from the refrigerator, the dew-point temperature of the air is above the temperature in the refrigerator. The Dew Point temperature can be measured by filling a metal can with water and ice cubes. Stir by a thermometer and watch the outside of the can. When the vapor in the air starts to condensate on the outside of the can, the temperature on the thermometer is pretty close to the dew point of the actual air. The Dew Point is given by the saturation line in the psychrometric chart. 2.3.4 Humidity Ratio or Moisture content Specific Humidity is the water vapor content of air, given in grams of water vapor per kg of dry air (i.e., kg of moisture/kg of dry air). It is also known as moisture content or humidity ratio. Air at a given temperature can support only a certain amount of moisture and no more. This is referred to as the saturation humidity. Humidity ratio is represented on the chart by lines that run horizontally and the values are on the right hand side (Y-axis) of the chart increasing from bottom to top. 28 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians Figure 2.7 Humidity Ratio or Moisture content 2.3.5 Specific Air Volume Specific Volume is the volume that a certain weight of air occupies at a specific set of conditions. The specific volume of air is basically the reciprocal of air density. As the temperature of the air increases, its density will decrease as its molecules vibrate more and take up more space (as per Boyle’s law). Thus the specific volume will increase with increasing temperature. Since warm air is less dense than cool air which causes warmed air to rise. This phenomenon is known as thermal buoyancy. By similar reasoning, warmer air has greater specific volume and is hence lighter than cool air. The specific volume of air is also affected by humidity levels and overall atmospheric pressure. The more the moisture vapor present in the air, the greater shall be the specific volume. With increased atmospheric pressure, the greater the density of the air - so the lower its specific volume. The unit of measure used for specific volume is cubic meter / kg of dry air. Specific volume is represented on Psychrometric Chart by lines that slant from the lower right hand corner towards the upper left hand corner at a steeper angle than the lines of wet bulb temperature and enthalpy. Psychrometry 29 Figure 2.8 Specific Air Volume 2.3.6 Sensible Heat Ratio (SHF) Figure 2.9 Sensible Heat Ratio Sensible Heat, Qs Sensible Heat Ratio = ---------------------------------------------Sensible Heat, Qs + Latent Heat, QL 30 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians The sensible heat ratio helps to determine the percentage of sensible heat and latent heat contribution to the total cooling load. ASHRAE psychrometric chart uses a protractor to plot the slope of the line representing the sensible heat ratio. Figure 2.10 Sensible Heat RatioProtractor 2.3.7 Relative Humidity (RH) Relative humidity (RH) is a measure of the amount of water air can hold at a certain temperature. temperature (dry-bulb) is important because warmer air can hold more moisture than cold air. Air Lines of constant relative humidity are represented by the curved lines running from the bottom left and sweeping up through to the top right of the chart. The line for 100 percent relative humidity, or saturation, is the upper, left boundary of the chart. Figure 2.11 Relative humidity Psychrometry 31 2.3.8 Enthalpy Enthalpy is the measure of heat energy in the air due to sensible heat or latent heat. Sensible heat is the heat (energy) in the air due to the temperature of the air and the latent heat is the heat (energy) in the air due to the moisture of the air. The sum of the latent energy and the sensible energy is called the air enthalpy. Enthalpy is expressed in Btu per pound of dry air (kilojoules per kilogram (kJ/kg). Enthalpy is useful in air heating and cooling applications. Air with same amount of energy may either be dry hot air (high sensible heat) or cool moist air (high latent heat).The enthalpy scale is located above the saturation, upper boundary of the chart. Lines of constant enthalpy run diagonally downward from left to right across the chart; follow almost exactly the line of constant wet bulb temperature. The enthalpy of moist air, in kJ/kg, is therefore: h = (1.007*t - 0.026) + g*(2501 + 1.84*t) Where g is the water content in kg/kg of dry air Figure 2.12 Enthalpy 32 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians 2.3.9 Combination of properties The chart below is the complete chart combining most of the lines and other parameters so far discussed: Figure 2.13 Combination of properties 1. Represents Sensible Heating 2. Sensible Heating and Humidification 3. Chemical Dehydration 4. Sensible Cooling 2.4 5. Cooling & dehumidification 6. Evaporative cooling 7. Latent heat addition-Humidification 8. Latent heat removal-Dehumidification Psychrometric processes The psychrometric process happens when the air at an initial state transforms and changes to final state. The transformation of air undergoes four basic processes (a) Sensible heating only (Heat addition into the air takes place without altering the moisture content) (b) Sensible cooing only (Heat removal from the air takes place without altering the moisture content) (c) Humidification only (Latent energy addition-Latent heating only-No change in dry-bulb temperature) (d) Dehumidification only (Latent energy removal-Latent cooling only-No change in dry bulb temperature) Figure 2.14 Four Basic Processes Psychrometry 33 In general the above four process involves the phase changes in water content, which are represented by the following figure: Figure 2.15 The Phase Change of water There are other processes involving both heat and water vapor transformation too, and they are classified as: Single processes (a) (b) (c) (d) (e) (f) (g) (h) Cooling and Dehumidification process (involving coils in air washer with chilled spray of water) Evaporative cooling process (Involving adiabatic process called Sensible cooling and humidification-Constant wet-bulb temperature) Water spray process Chemical Dehumidification process (Involving chemical or sorbent materials in adiabatic dehumidification-Constant wet-bulb temperature) Mixing of two air stream (Involving adiabatic process with no heat transfer) Room effect (Changes to supply air due to sensible and latent heat gains in the room) Fan Heat (Including fan, motor and drive(Similar to a sensible heating process-No change in water vapor) Enthalpy Wheel (Mixing process) Two or more processes in sequence (a) (b) (c) (d) (e) (f) Face & Bypass of mixing air-2 process in sequence Return Air Face & Bypass Reheat with cool and Dehumidification Sensible Precooling followed by Evaporative cooling Sensible Heating followed by Humidification Typical air conditioning cycle Now let us consider the first four basic processes 34 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians 2.4.1 Sensible Heating only Figure 2.16 Sensible Heating only This is the process where the temperature of the air stream is increased without any change in moisture content or specific humidity. The transfer heat into the air stream is done by one of the following devices: (a) Steam coil (b) Hot water coil (c) Heat pipe (d) Air-to-air heat exchanger (e) Sensible only rotary heat wheel. (f) Electrical heating coil (g) Furnace 2.4.2 Latent Heating Process A latent heating process occurs when water is evaporated without changing the dry bulb temperature. This is shown as vertical line in psychrometric chart. Figure 2.17 Latent Heating only Psychrometry 35 2.4.3 Sensible Cooling only process (Cooling without change in water vapor content) The transfer of heat from air using one of the following devices: (a) Chilled water (b) Refrigerant cooling coil (c) Indirect evaporative cooler (d) Heat pipe (e) Air-to-air heat exchanger (f) Sensible only rotary heat wheel (g) Air washers On a psychrometric chart, the sensible cooling process proceeds horizontally to the left along a line of constant humidity ratio towards the saturation line. In this process, there is no change in dew-point temperature, water vapor pressure, or humidity ratio. The heating and cooling explained above are represented on psychrometric charts as shown in the following figures. 2.4.4 Figure 2.18 Figure 2.19 Heating Cooling Heating and Humidification In this process, the air first passes through a heating coil and then through the humidifier where steam at a mass flow rate of required value and specific enthalpy hx is sprayed into the air stream. The heating and humidification of the air can be considered as two separate processes in sequence. Figure 2.20 Heating and Humidification Psychrometric chart 36 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians Referring to the psychrometric chart above, from point 1 to 2, the air passes through the heating coil and the sensible heat transfer takes place without altering the moisture content. From point 2 to 3, the moisture is added and the humidification of air occurs. During the sensible heating process of the moist air, the energy added is calculated by the following equation: Q = ma (h2 – h1) Where: Q = Rate of energy added, KJ/hr ma = mass flow rate of dry air through the process h2 = Specific enthalpy of moist air downstream of heating coil h1 = Specific enthalpy of moist air upstream of heating coil During the humidification process, the energy equation is; ma (h3 – h2) = mw hw Where: h3 = The specific enthalpy of the moist air downstream of the humidifier h2 = Specific enthalpy of moist air upstream of the humidifier hw = Specific enthalpy of the steam mw = Mass flow rate of the steam The rate of moisture addition to the air, mw, is determined by a water vapor mass balance mw = ma (w3 – w2) Where: w2 = Humidity ratio of the moist air upstream of the humidifier w3 = Humidity ratio of the moist air downstream of the humidifier Combining the equations, ma (h3 – h2) = mw hw and mw = ma (w3 – w2) ma (h3 – h2) = ma (w3 – w2) x hw h3 – h2 --------- = hw w3 – w2 Where the left hand side of the equation represents the slope of the humidification process on a psychrometric chart. Thus the direction of the process can be determined from the enthalpy of the steam added to the air stream and the enthalpy-Moisture protractor on a psychrometric chart. The specific humidity of air can also be increased by the injection of a predetermined quantity of steam into the air. It is important here that the steam is dry and saturated and there is no condensation at all. It is not possible to spray steam below 100ºC (at atmospheric pressure) as it is necessary to spray steam though the nozzles, which require higher pressure than atmospheric. Hence the lowest possible enthalpy carried with steam is the total heat of steam at 100ºC when the steam is fully dry and saturated. The amount of steam sprayed per kg of air is given by (W2 – W1). Psychrometry 37 2.4.5 Cooling and Dehumidification The removal of water vapor from air is termed dehumidification. It is only possible when the air is cooled below its dew point temperature. For effective dehumidification, it is necessary to maintain the cooling coil surface below the dew point temperature of air. Figure 2.21 Cooling and Dehumidification Let us take an example: Air is to be cooled from 35ºC DB and 24ºC WB to 20ºC DB and 17.6ºC WB. Take a psychrometric chart and mark these values. If we join these two points and draw a parallel line from the reference point to intersect the sensible heat factor line, we will notice that it intersects at 0.74 indicating that there is 26% latent heat removal and 74% sensible heat removal. The process of cooling and dehumidification is represented in the chart as follows: The cooling and dehumidifying process is shown in the psychrometric chart below. It begins at point 1 and ends at point 2. The refrigeration capacity required to accomplish this QR, is obtained from the energy balance. Energy Balance ma h1 = QR + ma h2 + mw hw Mass flow value for the water in the air ma W1 = mw + ma W2 Combining the above two equations QR = ma (h1-h2) – ma (W1-W2) hw 38 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians Figure2.22 Cooling and Dehumidification Psychrometric chart Where hw is the enthalpy of saturated liquid at temperature t2. The second term in the square bracket is the enthalpy associated with the liquid condensate as it runs out of the cooling coil. This term is small compared to (h1-h2) which is the enthalpy difference to cool the air and condense the water. The approximation is often made where the process is divided into sensible (S) and latent (L), components. QRS = ma (h2 – ha) and QRL = ma (ha – h1) Then QR = QRS + QRL QRS The sensible heat ratio for the process is then, SHR = --------------------------QRS + QRL The rate at which the moisture removed from the air is: mw = ma (W1 – W2) 2.4.6 Cooling with adiabatic humidification of air In this process, air is passed over a spray chamber. A spray chamber is a chamber with nozzles, which spray water. The temperature of the spraying water is more than the WBT of entering air and below the temperature of air. When air passes over this chamber, part of the water evaporates and is carried away by the air, increasing the specific humidity of air as shown in the figure below. Psychrometry 39 Figure 2.23 Cooling with Adiabatic saturation Air provides the heat required for the evaporation of water. During this time, the temperature of air decreases keeping the total enthalpy constant. Generally, complete humidification of the air in not possible thus the effectiveness of the spray chamber can be defined as: E = T1 – T3/(T1 – T2) Where: (T1 – T3) is the actual drop in the DBT (T1 – T2) is the ideal drop in DBT The humidifying efficiency is given by; efficiency = 100 × E. 2.4.7 Adiabatic chemical dehumidification When the high humid air is passed over a solid absorbent bed or a liquid absorbent spray, part of the water vapor will be absorbed reducing the water content in the air. The latent heat released is absorbed by air increasing its DBT and the total enthalpy remains constant. Thus the chemical dehumidification process follows the path along the constant enthalpy line. The effectiveness of the dehumidifier is defined as: E = (T3 – T1)/(T2 – T1) 2.4.8 Evaporative Cooling Systems The evaporative cooling can be classified as: 1. Direct evaporative system 2. Indirect evaporative system 3. Multi-stage evaporative systems 2.4.8.1 Direct evaporative system The figure below shows the schematic of an elementary direct, evaporative cooling system and the process on a psychrometric chart 40 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians Figure 2.24A Direct Evaporative Cooling system As shown in the figure, the direct evaporative cooling, the conditioned air comes in direct contact with the wetted surface, and gets cooled and humidified. In this process: a. The hot and dry outdoor air is first filtered and then is brought in contact with the wetted surface or spray of water droplets in the air washer. b. The air gets cooled and dehumidified due to simultaneous transfer of sensible and latent heats between air and water (process o-s). c. The cooled and humidified air is supplied to the conditioned space, where it extracts the sensible and latent heat from the conditioned space (process s-i). d. Finally the air is exhausted at state i. In an ideal case when the air washer is perfectly insulated and an infinite amount of contact area is available between air and the wetted surface, then the cooling and humidification process follows the constant wet bulb temperature line and the temperature at the exit of the air washer is equal to the wet bulb temperature of the entering air (to,WBT), i.e., the process becomes an adiabatic saturation process. However, in an actual system the temperature at the exit of the air washer will be higher than the inlet wet bulb temperature due to heat leaks from the surroundings and also due to finite contact area. Psychrometry 41 One can define the saturation efficiency or effectiveness of the evaporative cooling system ε as: Where, ε to tS to,WBT = Saturation efficiency = Outside air entering temperature = Supply air to conditioned space after evaporative cooling = Saturated wet-bulb temperature The amount of supply air required can be obtained by writing energy balance equation for the conditioned space, i.e. Where, mS Qt hi hS = The amount of supply air = The Total heat transfer rate (QS+Ql) = Enthalpy of return or exhaust air = Enthalpy of supply air Advantages (a) Compared to the conventional refrigeration based air conditioning systems, the amount of airflow rate required for a given amount of cooling is much larger in case of evaporative cooling systems. (b) The evaporative coolers are very useful essentially in dry climates Disadvantages (a) The evaporative coolers cannot provide comfort as the cooling and humidification line lies above the conditioned space condition ‘i’. (b) For a given outdoor dry bulb temperature, as the moisture content of outdoor air increases, the required amount of supply air flow rate increases rapidly (c) The conventional refrigeration based air conditioning systems can be used in any type of climate. 2.4.8.2 Indirect evaporative cooling system: The figure below shows the schematic of a basic, indirect evaporative cooling system and the process on a psychrometric chart. As shown in the figure, in an indirect evaporative cooling process, two streams of air - primary and secondary are used. Stream-1-The primary air stream becomes cooled and humidified by coming in direct contact with the wetted surface (o-o’), Stream-2-The secondary stream which is used as supply air to the conditioned space, decreases its temperature by exchanging only sensible heat with the cooled and humidified air stream (o-s). The moisture content of the supply air remains constant in an indirect evaporative cooling system, while its temperature drops. Obviously, everything else remaining constant, the temperature drop obtained in a direct evaporative cooling system is larger compared to that obtained in an indirect system, in addition the direct evaporative cooling system is also simpler and hence, relatively inexpensive. However, since the moisture content of supply air remains constant in an indirect 42 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians evaporation process, this may provide greater degree of comfort in regions with higher humidity ratio. The commercially available indirect evaporative coolers have saturation efficiency as high as 80%. Figure 2.24B Indirect Evaporative Cooling system 2.4.8.3 Multi-stage evaporative cooling systems: Figure below shows a typical two-stage evaporative cooling system and the process on a psychrometric chart. As shown in the figure, in the first stage the primary air cooled and humidified (o -o’) due to direct contact with a wet surface cools the secondary air sensibly (o -1) in a heat exchanger. In the second stage, the secondary air stream is further cooled by a direct evaporation process (1-2). Thus in an ideal case, the final exit temperature of the supply air (t2) is several degrees lower than the wet bulb temperature of the inlet air to the system (to). Psychrometry 43 Figure 2.25 Two-stage Evaporative Cooling system To improve efficiency of the evaporative cooling systems first sensibly cool the outdoor air before sending it to the evaporative cooler by exchanging heat with the exhaust air from the conditioned space. This is possible since the temperature of the outdoor air will be much higher than the exhaust air. It is also possible to mix outdoor and return air in some proportion so that the temperature at the inlet to the evaporative cooler can be reduced, thereby improving the performance. For example, one can use multistage evaporative cooling systems and obtain supply air temperatures lower than the wet bulb temperature of the outdoor air. Thus multistage systems can be used even in locations where the humidity levels are high. 2.5 Air-conditioning systems- Summer and Winter There are two basic systems in air-conditioning: • Summer air-conditioning systems • Winter air-conditioning systems Lets us now briefly study the various methods used for the above air-conditioning systems. 44 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians 2.5.1 Summer air-conditioning systems Summer air-conditioning system for hot and dry outdoor conditions As the name suggests these systems are used for hot and dry atmospheric conditions like temperature of 38–42°C and relative humidity of about 20–25%. In this process our purpose would be to reduce the air temperature and increase its relative humidity where the required comfort conditions are 24ºC and 60% RH. The general arrangement of the equipment and the psychrometric process are represented in the figures below. Figure 2.26 Summer air conditioning system for hot and dry outdoor conditions Figure 2.27 Representation of psychrometric process Atmospheric air is passed through the dampers and gets filtered before passing over the cooling coil. When the air is passed over the cooling coil, its temperature is reduced by sensible cooling as represented by point 2 on the psychrometric process chart. Psychrometry 45 The air coming out from the cooling coil at point 2 is passed into an adiabatic humidifier where the water vapor increases the humidity of air and the conditioned air leaves the humidifier at point 3. The efficiency of the humidifier is given by the equation: Efficiency = [(T2 – T3) /(T2 – T5 )] × 100 If the quantity of atmospheric air supplied is V L/sec, then the capacities of the cooling coil and the humidifier are given by: Total capacity of cooling coil = (V / Hf) × [( h3 – h1)/1000] KW of refrigeration Where: V is the volume of handled air in L/sec Hf is the density of moist air Kg/m3. Capacity of humidifier = (V / Vs) × [(w3 – w2) / 1000] kg/sec Summer air-conditioning system for hot and humid outdoor conditions As the name suggests these systems are used for hot and humid atmospheric conditions like; temperature of 32–38°C and relative humidity of about 70–75 %. Figure 2.28 Summer air conditioning system for hot and wet weather In this process our purpose would be to reduce the air temperature and its relative humidity where the required comfort conditions are the same: 24ºC and 60 % RH. The general arrangement of the equipment and the psychrometric process are represented in the following figures. Figure 2.29 Representation of psychrometric process 46 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians Here the air is filtered and then passed over the cooling coil for dehumidification. As air is passed over the cooling coil whose temperature is below the dew point temperature of incoming air, the temperature and humidity of air is reduced and it comes out at point 3. The capacity of the cooling coil is given by the equation; Total capacity of cooling coil = Hf×(V) × [( h1 – h3 ) / 1000] KW of refrigeration The air then enters the heating coil condition 3 and leaves at condition 5 Capacity of heating coil = Hf × (V) × (h5 – h3)/1000 KW Summer air-conditioning system with single cooling coil and mixing This type of system is used to reduce the load on the cooling coil as part of the air going out of the room, which at a lower temperature than the outdoor condition, is mixed with fresh air. The arrangement of the system is shown in figure and the corresponding processes are represented on the psychometric chart Figure 2.30 Arrangement for the components for the given air-conditioning system Figure 2.31 Psychrometric process for the given system Psychrometry 47 Condition (4) is the mixing of air at conditions (2) and (3). Condition (5) is the condition of air leaving the cooling coil and 5–1 represents the heating of air passing through the blower due to friction. The process 1–2 represents the condition of air passing through the air-conditioned room taking the load in the room. The details of the cooling system (refrigeration) used in single coil direct expansion system are shown in following Figure. Figure 2.32 Direct expansion refrigeration system for cooling and dehumidifying of hot and moist air This system is known as a direct expansion system as the refrigerant is directly used for cooling the air in the evaporator. But in large systems, used for comfort air-conditioning and having several cooling coils, a centrifugal refrigeration plant processes chilled water and chilled water is further circulated to the various cooling coils. This system is known as an indirect cooling system. A centrifugal compressor using would be used for producing chilling water, as it has to handle a large quantity of refrigerant. Summer air-conditioning with single coil and bypass mixing This system is used to control DBT in the air-conditioned room as per the load in the room. The arrangement of the system is shown in Figure. 48 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians Figure 2.33 Arrangement for the components for the given air-conditioning system Condition 4 is the mixing of air at conditions 2 and 3. Condition 5 is the condition of air coming out of the cooling air. Condition 6 is the mixing of air at conditions 5 and 2. Process 4–5 represents the cooling and dehumidifying of air passing through the cooling coil. Process 6-1 is heat generated by fan and motor. Process 1–2 represents the condition of air passing through the room as it takes the load in the room. The re-heating of air passing through the blower due to friction is neglected for plotting on the psychrometric chart. The previous system used has some limitations, as the temperature in the air conditioned room cannot be controlled according to the load in the room. The control of DBT is more important than humidity control as long as humidity is not excessive. The present system is used during partial load operation. The face dampers on the cooling coil and bypass dampers are controlled by a motor, which positions them so as to maintain a constant DBT. As the sensible heat gain of the air-conditioned space decreases, more re-circulated air is bypassed. However with direct expansion cooling coil, the air, which passes across the coil, may be more thoroughly dehumidified than when the full air quantity is handled. Thus satisfactory space humidity conditions may be maintained during some partial load conditions without the need for re-heating. Summer air-conditioning with single cooling coil and absorbent dehumidifier: The cooling coil discussed in the above methods for cooling the air, also produces some dehumidification in conjunction with the cooling process. The dehumidification of air by a refrigerant cooling coil has limitations. If the coil surface temperature is less than 0°C, frost forms on the coil and the heat transfer rate reduces. A defrosting system is required and reheating of the air is needed before passing into the air-conditioned space. This refrigeration system becomes more complicated and more expansive to own and operate as the required air dew point temperature is reduced. The absorbent system shown in the following figure can reduce the required surface temperature of the cooling coil and completely avoids the possibility of frosting the coil as the required coil temperature is always above 0°C. Therefore this method produces extremely low air dew-point temperatures, more reliably and more economically than the refrigeration method. The psychrometric processes for the above-described system are shown in the following Figure. Psychrometry 49 Figure 2.34 Arrangement for the components for the given air-conditioning system Figure 2.35 Psychrometric Processes For Given System Condition 4 is the mixing of airs at conditions 2 and 3. Process 4–5 represents the adiabatic dehumidification of air passing through the absorbent dehumidifier. Process 5–6 is the sensible cooling of air passing through the cooling coil whose surface temperature is considerably above the temperature required for frosting. Process 6–1 is heat generated by fan and fan motor. Process 1–2 is the condition of air passing through the air-conditioned room, taking the existing load. Summer air-conditioning with evaporating cooling: Comfort air-conditioning systems capable of maintaining optimum thermal conditions may be expensive to own and operate. Partially effective systems, which involve much less costs, may be attractive where finances preclude the installation of a completely effective system. In hot dry regions evaporating cooling systems may be capable of providing considerable relief in enclosed spaces. The evaporative cooling system commonly used is shown in Figure below and the corresponding processes. 50 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians Figure 2.36 Arrangement for the components for the given air-conditioning system Process 3–1 represents evaporative cooling and process 1–2 represents room load taken by the air passing through the room. State 2 is an acceptable space condition although not necessarily an optimum one. State 3 of the outdoor air is at a much higher temperature but lower RH than state 2. As the air-washer is the only processing device in the system, the cost of the system is considerably lower than the system used for optimum comfortable conditions. Generally, a much higher flow rate of air is used with an evaporative cooling system (2 to 3 times of conventional) than with conventional systems. A high rate of air movement past a person allows the same degree of comfort but with higher effective temperatures as compared to the situations where airmovement is low. 2.5.2 Winter air-conditioning systems The required comfort air conditions are the same as in summer. The typical arrangement of the required equipment and its representation on the psychrometric chart are shown in the following Figure below. Figure 2.37 Winter air conditioning system Psychrometry 51 Air is passed through the resistance heater known as the preheating coil, and then through the humidifier. It is then passed through the second preheating coil. Winter air-conditioning with double reheat coils and air washer During severe winter conditions it is always necessary to increase the DBT and RH of the air. The arrangement of the components in this system and its representation on the psychrometric chart are shown in the following figures. Figure 2.38 Arrangement for the components for the given air-conditioning system Condition 4 is the mixing of air at conditions 2 and 3. Process 4–5 is the sensible heating in the preheat coil. Process 5–6 is the adiabatic cooling of the air passing through the air washers and process 6–1 is the sensible heating in the reheat coil. Process 1–2 is the cooling and dehumidifying of the air passing through the conditioned room. This compensates for the heat and the vapor loss of the air in the conditioned room. In large systems it is a common practice to use re-circulating air fans as well as supply air fans. However this condition does not affect the process represented in the above psychrometric chart. Winter air-conditioning using 100% outdoor air with pre-heating (by waste heat of the exhaust) In designing any air-conditioning system every effort has to be made to utilize internal heat emission wherever economically feasible. Such a system is shown in the following figure where the waste heat from the exhaust is used for preheating fresh air. Figure 2.39 Arrangement for the components for the given air-conditioning system 52 Practical Fundamentals of Heating, Ventilation & Air-conditioning (HVAC) For Engineers and Technicians The air washers serve as humidifying devices to offset the moisture losses in the air conditioned space and in addition to this, it cleans the air. The reheat coil regulates the heat supply thus controlling the DBT of the air-conditioned space as required. Process 4–5 is the preheating of fresh air by using the waste heat in the exhaust air. Here process 2–3 shows the cooling of exhaust air. Process 5–6 is the humidification of air by using steam and process 6–1 is the sensible heating in the reheat coil. Process 1–2 is the cooling and dehumidification of air to compensate for the heat and vapor loss in the conditioned space. In winter air-conditioning systems where heating is required, the use of outdoor air should be kept to a minimum.