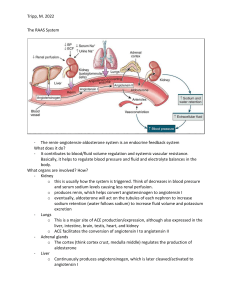
SODIUM REGULATION HEMOSTASIS Nima Behmard 01-76 Na+ BALANCE o Major cation of ECF o All these mentioned factors are related to each other. Amount of Na+ in ECF Volume of ECF Plasma volume Blood volume Blood pressure ALDOSTERONE o mineralocorticoid steroid hormone o primary function is to elevate blood pressure o Aldosterone's primary target is the nephron, the functional unit of the kidney o release from glomerulosa cells (GC) in the cortex of the adrenal glands: • extracellular K+ concentration • the renin-angiotensin system (RAS) Renin o An aspartic protease that hydrolyzes the liver-released proenzyme angiotensinogen to produce angiotensin I → cleaved further by carboxypeptidase angiotensin-converting enzyme (ACE) to produce active angiotensin II (ANG II). o ANG II induces the GC to produce aldosterone. o Klotho protein (KL), ACTH, natriuretic peptides (NPs), and the circadian clock are all capable of regulating aldosterone production o These are causes juxtaglomerular cells (JGC) in the afferent arteriole of the nephron to release renin into the circulation • • • Reduced blood pressure The sympathetic nervous system reduced NaCl supplied to the distal tubule o When the juxtaglomerular apparatus's macula densa cells detect a low NaCl content in the filtrate → they release paracrine signals that activate JGC. Angiotensin II o Angiotensin II is the primary signal for increased aldosterone secretion by adrenal glomerulosa cells o Actions on the kidney o By constricting renovascular smooth muscle it increases vascular resistance in the kidney and hence decreases renal blood flow and glomerular filtration o Therefore, by decreasing glomerular filtration, angiotensin II decreases sodium and water excretion Angiotensin II o Cardiovascular effects • Stimulation of angiotensin II receptors in vascular smooth muscle results in • increased intracellular calcium concentrations and sustained vasoconstriction Acts directly on cardiac myocytes to increase calcium influx and therefore cardiac contractility o Central nervous system effects • Angiotensin II, acting both as a hormone and as a neurotransmitter, stimulates thirst, appetite for sodium, and secretion of ADH through actions exerted on the hypothalamus Angiotensin II Angiotensin II Mechanisms of Aldosterone Secretion o the primary signaling molecules that govern aldosterone synthesis are o ANG II, ACTH, and K+ o These inputs can function in two ways: acute and chronic. The acute reaction o occurs within minutes o leads in an increase in aldosterone owing to the activation of enzymes involved in the o biosynthesis process the mobilization of cholesterol whereas the chronic reaction o impact occurs hours later o includes changes in gene expression Aldosterone Biosynthesis Pathway o Aldosterone is synthesized in the mitochondria and endoplasmic reticulum of zona glomerulosa cells in the adrenal cortex. o The cholesterol precursor can be derived from a combination of sources: o mobilization of cholesteryl esters stored in lipid droplets by cholesteryl ester hydrolase o de novo synthesis in the endoplasmic reticulum o receptor-mediated uptake o internalization of plasma lipoprotein-derived cholesterol. Aldosterone Biosynthesis Pathway o The free cholesterol is transported by the steroidogenic acute regulatory (StAR) protein from the outer to the inner mitochondrial membrane, which is the early rate-limiting step in steroidogenesis. o In the inner mitochondrial membrane, steroidogenesis is initiated by the side-chain cleavage of cholesterol catalyzed by CYP11A1 to yield the steroid precursor, pregnenolone. o Pregnenolone passively diffuses to the endoplasmic reticulum where it is converted to progesterone by type II 3β-hydroxysteroid dehydrogenase (3βHSD2). o Progesterone is then hydroxylated to 11-deoxycorticosterone by CYP17. o The final late rate-limiting steps of aldosterone biosynthesis are completed in the mitochondria, where aldosterone synthase (CYP11B2) catalyzes the conversion of 11deoxycorticosterone to corticosterone and subsequently to aldosterone. Aldosterone Biosynthesis Pathway Adrenocorticotropin hormone o ACTH (Adrenocorticotropin hormone) from the pituitary gland stimulates the adrenal cortex. o After stimulation of the adrenal cortex by the ACTH, the process of steroidogenesis starts from the cholesterol. o The pituitary gland (ACTH) is stimulated by the Hypothalamic hormone (Corticotropinreleasing factor (CRH). o ACTH initially enhances GC cell aldosterone synthesis; however, with continued induction by ACTH, the GC phenotype shifts to that of zona fasciculata, resulting in a reduction in aldosterone synthesis. Adrenocorticotropin hormone o Cellular mechanisms involved in the biosynthesis of adrenal steroids. The cellular mechanisms that underlie adrenal steroidogenesis are initiated by activation of the adrenocorticotropic hormone (ACTH) receptor and angiotensin II type I (ATIIR) receptor by ACTH and angiotensin II, respectively. Steroidogenesis is also initiated by changes in serum potassium levels through the specific expression of potassium channels in the zona glomerulosa. This is followed by the activation of second messengers, which initiate cholesterol mobilization. Cholesterol can be obtained from LDL and HDL lipid carriers, lipid droplets, or de novo synthesis. Unesterified cholesterol is transported to the mitochondria, where cleavage by CYP11A1 is the first step of steroidogenesis. Tissuespecific expression in the various zones of the adrenal cortex leads to aldosterone and cortisol biosynthesis. Abbreviations: 3β HSD, 3β-hydroxysteroid dehydrogenase; HSL, hormone-sensitive lipase; LDLR, low-density lipoprotein receptor; SR-BI, scavenger receptor class BI. Adrenocorticotropin hormone Klotho protein o is a single-pass transmembrane type 1 glycoprotein o has been identified as an anti-aging molecule since serum KL levels decline with age. o Decreased serum KL levels have been linked to age-related diseases include • coronary artery • disease, • atherosclerosis, • myocardial infarction, • and hypertension. o In mice, there was a negative connection between serum KL and aldosterone levels. Natriuretic peptides (NPs) o o o o o o o are primarily released by the heart aid in vasodilation and fluid regulation. can operate as endocrine components and have autocrine and paracrine signaling capacities. They have been postulated to control aldosterone secretion because to their function in blood pressure regulation. peptides in crude cardiac extracts were able to suppress GC aldosterone synthesis even when stimulated by ANG II and ACTH. atrial NPs inhibit the aldosterone response to ANG II in rats. Human men experienced similar outcomes. Natriuretic peptides (NPs) o o o o o The simultaneous injection of modest amounts of atrial NPs with either ACTH or ANG II had no effect on blood pressure or aldosterone levels. Another method NPs control blood pressure is through influencing renal filtration and renin release. Renin secretion was significantly reduced in isolated rabbit afferent arterioles and suspended JGC subjected to NPs. In vivo investigations in dogs support these findings, with atrial NP infusion increasing renal flow, glomerular filtration rate, salt and potassium excretion, while decreasing blood pressure and renin production. These findings imply that RAS and NPs may function as endogenous antagonists. Circadian clock o The molecular clock is comprised of a transcriptional loop in which heterodimers of the transcription factors BMAL1 (brain and muscle ARNT-like protein 1) and CLOCK (circadian locomotor output cycles kaput) stimulate the transcription of the Per and Cry genes. Accumulating PER and CRY proteins feedback to inhibit the BMAL1– CLOCK heterodimer. Rhythmic, antiphase expression of two nuclear receptors, ROR and REV-ERB, maintain the circadian pattern of gene expression of Bmal1 and Clock via RORE response elements. The output of the molecular clock is rhythmic expression of a wide range of genes, dubbed clock-controlled genes, that typically contain E-box (BMAL1/CLOCK), D-box (DBP or E4BP4) or ROR (ROR or REV-ERB) response elements and thus follow a circadian pattern of expression coupled to circadian oscillations of these transcription factors. Cheng et al.3 demonstrate that key genes regulating progression of proteins through the secretory pathway are CCG’s, allowing circadian regulation of secretion of proteins that are not direct CCG’s themselves. Circadian clock Circadian clock o o o o o Several physiological activities, including as blood pressure, immunological response, and metabolism, are possibly controlled by the circadian clock via four "circadian clock" proteins: • • • • period 1-3 (Per 1-3), Bmal1, clock cryptochrome 1-2, and Clock. Per1 controls ENaC expression in both an aldosterone-dependent and an aldosterone-independent way. also controls the expression of other genes involved in renal Na+ reabsorption in a coordinated manner. They include Per1-mediated Na+-K+-ATPase upregulation via Fxyd5 and endothelin 1 downregulation, which is a possible ENaC inhibitor. Per-1 regulates not only the downstream targets of aldosterone, but also the plasma levels of aldosterone. Circadian clock o o o o o o Per1 knockout mice had lower levels of aldosterone and 3-dehydrogenase isomerase. Male mice appear to be more vulnerable to the negative phenotypes of Per-1 KO than female mice. Deoxycorticosterone pivalate (DOCP), an aldosterone analog, elevated mean arterial pressure and disrupted normal circadian blood pressure in Per-1 KO mice on a high salt diet. These effects are not seen in female Per-1 KO mice treated similarly. Endothelin 1 can explain this discrepancy. Male mice fed a high salt diet and given DOCP showed a lower night/day ratio of urine ET-1 and different ET-1 and ET-1 receptor gene expression than female mice. Circadian clock The baseline profile from the circadian study shows a trough in aldosterone during sleep and a peak in the morning upon awakening. Data were standardized to each participant's time of sleep and then averaged. Circadian clock Pathology • Disorders of plasma sodium are the most common electrolyte disturbances in clinical medicine, yet they remain poorly understood. Severe hyponatraemia and hypernatraemia are associated with considerable morbidity and mortality, however, and even mild hyponatraemia is associated with worse outcomes when it complicates conditions such as heart failure, although which is cause and which effect is often uncertain. Distinguishing the cause(s) of hyponatraemia may be challenging in clinical practice, and controversies surrounding its management remain. Pathology • • • • • • • Sodium imbalances are widespread, especially in hospital patients and the elderly. Mild sodium problems might be asymptomatic and self-limiting, while severe sodium disorders have a high morbidity and fatality rate. The reasons of sodium imbalance are frequently iatrogenic and hence preventable. Assessing hydration status and monitoring sodium levels in plasma and urine are critical steps in determining the etiology of hyponatraemia. The history will generally reveal the cause of hypernatraemia. For the treatment of salt problems, there is little evidence from randomized controlled studies. Slow sodium correction is typically safe, as long as clinical status and plasma sodium are closely monitored. Hyponatraemia • If a clear precipitating reason is present, such as vomiting or diarrhoea, when both sodium and total body water are low, and especially if the patient (usually elderly) is taking diuretics, determining the etiology of hyponatraemia may be easy. In hospital practice, determining the reason is frequently more difficult. Hyponatraemia nearly invariably reflects an excess of water relative to sodium, most typically as a consequence of dilution of total body sodium subsequent to increases in total body water (water overload), but sometimes occasionally as a result of total body sodium depletion in excess of contemporaneous body water losses. The clinical categorization of hyponatraemia based on the extracellular fluid volume status of the patient, as hypovolaemic, euvolaemic, or hypervolaemic. Classification of hyponatraemia • Hypovolaemia • Hypervolaemia • Extrarenal loss, urine sodium <30 mmol/l • Urine sodium <30 mmol/l • • Dermal losses, such as burns, sweating Gastrointestinal losses, such as vomiting, diarrhoea Pancreatitis Renal loss, urine sodium >30 mmol/l • • • • • Congestive cardiac failure Cirrhosis with ascites Nephrotic syndrome Urine sodium >30 mmol/l • Chronic renal failure Diuretics Salt wasting nephropathy Cerebral salt wasting Mineralocorticoid deficiency (Addison's disease) • Paradoxical retention of sodium and water despite a total body excess of each; baroreceptors in the arterial circulation perceive hypoperfusion, triggering an increase in arginine vasopressin release and net water retention. • • • • • • Classification of hyponatraemia • • • • • • • • • Euvolaemia Urine sodium >30 mmol/l Syndrome of inappropriate antidiuretic hormone secretion (SIADH) Hypothyroidism Hypopituitarism (glucocorticoid deficiency) Water intoxication: Primary polydipsia Excessive administration of parenteral hypotonic fluids Post-transurethral prostatectomy Hypernatraemia • Hyponatraemia is far more prevalent than hypernatraemia.3 It represents either a net water loss or a hypertonic sodium increase, with the resulting hyperosmolality. Only sudden and significant elevations in plasma sodium concentrations over 158-160 mmol/l cause severe symptoms. Importantly, in individuals with altered mental state or hypothalamic lesions affecting their feeling of thirst (adipsia), as well as babies and the elderly, the sensation of extreme thirst that protects against severe hypernatraemia may be absent or decreased. Non-specific symptoms such as anorexia, muscular weakness, restlessness, nausea, and vomiting are common in the early stages. More severe symptoms include changed mental state, lethargy, irritability, stupor, or coma. Acute brain shrinkage can result in vascular rupture, which can result in cerebral bleeding and subarachnoid hemorrhage. Classification of hyponatraemia • Hypovolaemia • Hypervolaemia • Dermal losses—for example, burns, sweating Gastrointestinal losses—for example, vomiting, diarrhoea, fistulas Diuretics Postobstruction Acute and chronic renal disease Hyperosmolar non-ketotic coma • Iatrogenic (hypertonic saline, tube feedings, antibiotics containing sodium, or hypertonic dialysis) Hyperaldosteronism - Typically mildly elevated sodium ∼147 mmol/l, so rarely a clinical problem • • • • • • • Euvolaemia • • • • • Diabetes insipidus (central, nephrogenic, or gestational) Hypodipsia Fever Hyperventilation Mechanical ventilation Sources • Agarwal, R. (2010). Regulation of circadian blood pressure: from mice to astronauts. Curr. Opin. Nephrol. Hypertens. 19, 51–58. doi: 10.1097/MNH.0b013e3283336ddb • Anantharam, A., and Palmer, L. G. (2007). Determination of epithelial Na+ channel subunit stoichiometry from single-channel conductances. J. Gen. Physiol. 130, 55– 70. doi: 10.1085/jgp.200609716 • Anderson, J. V., Struthers, A. D., Payne, N. N., Slater, J. D., and Bloom, S. R. (1986). Atrial natriuretic peptide inhibits the aldosterone response to angiotensin II in man. Clin. Sci. 70, 507–512. • Arima, S., Kohagura, K., Xu, H. L., Sugawara, A., Abe, T., Satoh, F., et al. (2003). Nongenomic vascular action of aldosterone in the glomerular microcirculation. J. Am. Soc. Nephrol. 14, 2255–2263. doi: 10.1097/01.ASN.0000083982.74108.54 Sources • Arriza, J. L., Weinberger, C., Cerelli, G., Glaser, T. M., Handelin, B. L., Housman, D. E., et al. (1987). Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237, 268– 275. • Arteaga, M. F., Wang, L., Ravid, T., Hochstrasser, M., and Canessa, C. M. (2006). An amphipathic helix targets serum and glucocorticoid-induced kinase 1 to the endoplasmic reticulum-associated ubiquitin-conjugation machinery. Proc. Natl. Acad. Sci. U. S. A. 103, 11178–11183. doi: 10.1073/pnas.0604816103 • Reynolds, R. M., Padfield, P. L., & Seckl, J. R. (2006). Disorders of sodium balance. Bmj, 332(7543), 702-705.