Corrosion & Metal Plating: Cathodic Protection, Electroless Plating
advertisement
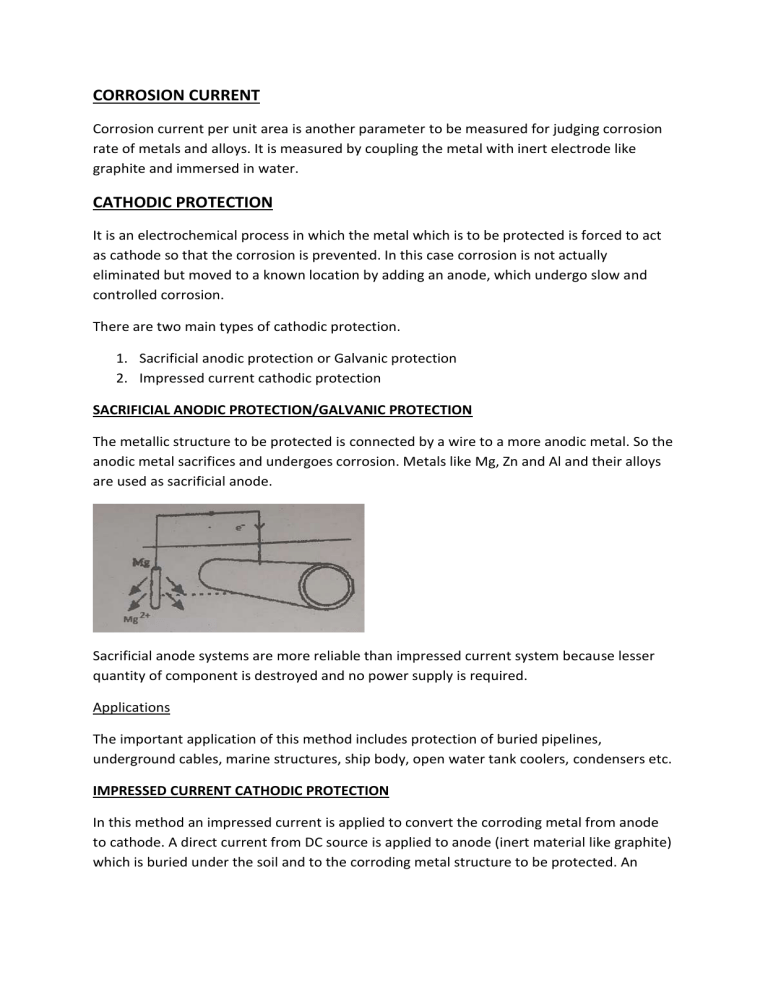
CORROSION CURRENT Corrosion current per unit area is another parameter to be measured for judging corrosion rate of metals and alloys. It is measured by coupling the metal with inert electrode like graphite and immersed in water. CATHODIC PROTECTION It is an electrochemical process in which the metal which is to be protected is forced to act as cathode so that the corrosion is prevented. In this case corrosion is not actually eliminated but moved to a known location by adding an anode, which undergo slow and controlled corrosion. There are two main types of cathodic protection. 1. Sacrificial anodic protection or Galvanic protection 2. Impressed current cathodic protection SACRIFICIAL ANODIC PROTECTION/GALVANIC PROTECTION The metallic structure to be protected is connected by a wire to a more anodic metal. So the anodic metal sacrifices and undergoes corrosion. Metals like Mg, Zn and Al and their alloys are used as sacrificial anode. Sacrificial anode systems are more reliable than impressed current system because lesser quantity of component is destroyed and no power supply is required. Applications The important application of this method includes protection of buried pipelines, underground cables, marine structures, ship body, open water tank coolers, condensers etc. IMPRESSED CURRENT CATHODIC PROTECTION In this method an impressed current is applied to convert the corroding metal from anode to cathode. A direct current from DC source is applied to anode (inert material like graphite) which is buried under the soil and to the corroding metal structure to be protected. An impressed current is applied from the DC source which is in opposite direction to neutralize corrosion current to zero or negative. E.g.: ship docked in harbor are protected by this method. ELECTROLESS PLATING It is defined as a controlled autocatalytic deposition of a continuous layer of noble metal from its salt solution on a catalytic surface of the base metal by using a suitable reducing agent without using electrical energy. Process of electroplating: The process of electroless plating involves the following steps. Preparation of the surface to be plated: In this step the surface of the metal to be plated is activated by following steps. Etching: Removal of impurities by acid treatment Chemical Treatment: The plastic objects and printed circuit boards are treated with SnCl2 and PdCl2. This treatment yields a thin layer of Pd on the treated surface. Electroplating: A thin layer of the metal to be plated or any other suitable metal is coated on the surface of the object. Preparation of plating bath: The plating bath should include the following: Soluble salt of metal like chloride or sulphate solution. Complexing agents like EDTA, tartarate, citrate etc. to prevent excess deposition. Reducing agents like sodium thiosulphate, formaldehyde etc. Accelerators like succinates, glycinates, fluorides etc. to enhance the plating rate. Stabilizers like calcium ions, lead ions, thiourea etc. to prevent decomposition of the bath and to impart stability to the solution. Buffer solution like Rochelle salt, Boric acid to maintain pH. Procedure The article to be plated is immersed in the bath containing the metal salt and reducing agent. The plating is carried out in a series of tanks. The rate of deposition is controlled by the amount of reducing agent and the type of chelating agent. Stability of Plating The stability of electroless plating fully depends on the substrate material, the pretreatment process, the type of the solution used and the pH and temperature during plating. Advantages 1. Surface to be coated does not need to be conductive, thus electroless plating can be made on both metals and plastics. 2. Electrical power and other accessories are not required. 3. Gives uniform coating even on the objects with irregular shapes, holes recess etc. 4. Coating provides better wear resistance. 5. The coating is less porous and provide better corrosion resistance. 6. Provides flexibility in plating volume and thickness ELECTROLESS COPPER PLATING Electroless copper plating is used to deposit a layer of copper on the substrate without the use of electricity. It is commonly used for making printed circuit boards. Procedure: The object to be plated is degreased using organic solvent and this is followed by acid treatment. This object is then immersed in plating bath containing cupric salt, reducing agents, buffer and complexing agent. Reactions at cathode: Cu2+ + 2eCu At anode: 2HCHO + 4OH-2HCOO-+2H2O+H2+2e Uses: 1. used in double or multilayered printed circuit boards in which plating through holes is required. 2. used as base coating for subsequent electroplating. ELECTROLESS NICKEL PLATING Metals like Al, Fe, Cu and alloys like brass are plated with nickel. Non metallic materials like glass, quarts and plastics are pretreated with SnCl2 in acid medium followed by treatment with PdCl2solution. The pretreated object is immersed in the plating bath containing nickel salt and reducing agent like sodium hypophosphite for a certain time and nickel gets coated over the object. At cathode: Ni2+ + 2eNi At anode: H2PO2- + H2ONi + H2PO3- +2H+ As H+ are released in the reaction, the pH of the solution changes. To maintain the constant pH, buffer solutions are generally used. This gives the uniform fine-grained plating Uses 1. in aerospace compounds: for making wear resistance, corrosion protection, chemical resistivity and lubricity on valves pistons, engine shafts, engine mounts, compressor blades etc. 2. in packaging and handling machinery: due to its wear resistance, cleanliness and attractive finish. 3. In chemical manufacturing: due to chemical resistance used in chemical manufacturing and storage equipment. 4. In automotive components: for wear protection and corrosion resistance on pistons, cylinders, gears, different pinion ball shafts, fuel injectors, ball studs, heat sinks etc.



