Basic Biochemical Techniques and Microscopical Techniques report
advertisement

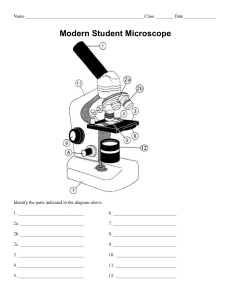
YILDIZ TECHNICAL UNIVERSITY DEPARTMENT OF BIOMEDICAL ENGINEERING EXPERIMENT 1 Basic Biochemical Techniques and Microscopical Techniques Author: MARWA ABOUARRA 19.October.2022 MERVE EBUARRA Contents: 1. Purpose of the Experiment 2. Introduction 3. Materials 4. Methods 5. Results 6. Discussion 7. References LIST OF FIGURES FIGURE 1.PERIODIC TABLE OF ELEMENTS [1] .............................................................................................................................................................................................. 1 FIGURE 2.UNIVERSAL PH INDICATOR.......................................................................................................................................................................... 3 FIGURE 3. LETTER E AT 4X UNDER THE MICROSCOPE ............................................................................................................................................. 6 FIGURE 4. LETTER E AT 10X UNDER THE MICROSCOPE ........................................................................................................................................... 6 FIGURE 5. LETTER E AT 40X UNDER THE MICROSCOPE ........................................................................................................................................... 6 LIST OF TABLES TABLE 1.COMMON UNITS OF CONCENTRATION [5] ................................................................................................................................................................................. 2 TABLE 2. CHART COMPARING ACIDS AND BASES [7]............................................................................................................................................................................. 3 TABLE 3. MICROSCOPE FUNCTION................................................................................................................................................................................. 4 TABLE 4. MATERIALS USED IN THE LAB ...................................................................................................................................................................... 4 Figure 1.Periodic Table of Elements [1] 1 MERVE EBUARRA 1. Purpose of the Experiment To learn how to; 2. i. make a buffer solution, ii. use the microscope and learn how its lenses functions’, iii. prepare a solution and find the pH using the pH meter. Introduction In chemistry, a solution is the result of mixing two or more compounds in relative proportions, and it is homogeneous. [2] These two or more compounds are referred to as solvent and solute. A solvent is usually in liquid form, which is the dissolving agent, while the solute is the substance that is being dissolved. [3] Although solutions of gases and solids are possible, the term "solution" is most frequently used to refer to the liquid condition of matter. Brass is a solution made of copper and zinc, whereas air is primarily an oxygen and nitrogen mixture with a little bit of concentrations from other gases. [2] Concentration is the process of separating rare earth minerals from the other materials derived from the grinding mill. This is performed in large-scale operations by making use of the various characteristics of the minerals that need to be separated. These characteristics can include magnetic or electric (magnetic and electrostatic separation), color (optical sorting), density (gravity separation), and physicochemical (flotation separation). [4] Concentration has units but the most used and known one is called molarity, which represent the quantity of moles of a solute existing in precisely 1L of solution. [5] Molarity equation 2.1 [5] 𝒎𝒐𝒍𝒂𝒓𝒊𝒕𝒚 = 𝒏 𝒎𝒐𝒍𝒆𝒔 𝒐𝒇 𝒔𝒐𝒍𝒖𝒕𝒆 𝑽𝒐𝒍𝒖𝒎𝒆 𝒐𝒇 𝒔𝒐𝒍𝒖𝒕𝒊𝒐𝒏 𝒏 = mol 𝑽 n: represent the number of moles in a solute. • Calculating Moles from Concentration of NaCl [5] Example 2.1: Calculate the number of moles of sodium chloride (NaCl) in 150 mL of 0.100 M NaCl. 0.1 𝑀 0.1 𝑀 15 1 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝐶𝑙 = 150𝑚𝐿 × = 0.15𝐿 × = × = 0.015 𝑚𝑜𝑙𝑒 𝑁𝑎𝐶𝑙 1𝐿 1𝐿 100 10 Table 1.Common Units of Concentration [5] Concentration m/m m/v Units 𝑔 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝑔 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 𝑔 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝑚𝐿 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 2 MERVE EBUARRA We can tell if something is acidic, alkaline, or neutral by using an indicator. Indicators are substances that show different colors when they are in acidic or alkaline conditions. Figure 2.Universal pH Indicator Litmus paper is a simple indicator that tells us whether something is acid or alkali. Litmus is red in acids and blue in alkalis. Litmus paper is made from lichens which have been used to die cloth for hundreds of years. It comes as red litmus paper and blue litmus paper. Red litmus paper changes color from red to blue under alkaline conditions but no change under acidic conditions. Blue litmus paper changes color from blue to red under acidic conditions but no change under alkaline conditions. However, litmus paper only tells us whether something is acid or alkali, but it does not tell us how acidic or alkaline a substance is. For this, we have the pH scale and a universal indicator. Universal indicator is a mixture of different indicators or dyes which has many different color changes and so shows us the pH value of the solution. The pH scale runs from 0 to 14. With 0 being extremely acidic, 7 being neutral and 14 being extremely alkaline. Stomach acid is a strong acid with pH of about 2. Acid rain has a pH of about 5.5. Milk is neutral with a pH of 7. Seawater is slightly alkaline. Soapy water is a strong alkaline and bleach is a strong alkaline with a pH of 13. Universal indicator is so called due to its ability to indicate the entire pH spectrum.[6] Some fun facts about the difference between acids and bases.[7] Table 2. Chart Comparing Acids and Bases [7] Characteristic reactivity pH taste (don't test unknowns this way) corrosivity touch (don't test unknowns) litmus test conductivity in solution common examples Acids accept electron pairs or donate hydrogen ions or protons pH< 7 sour Bases donate electron pairs or donate hydroxide ions or electrons 7 < pH soapy or bitter may be corrosive astringent may be corrosive slippery red conduct electricity vinegar, lemon juice, sulfuric acid, hydrochloric acid, nitric acid blue conduct electricity bleach, soap, ammonia, sodium hydroxide, detergent 3 MERVE EBUARRA We can also get even more accurate measurements of pH using a pH meter which will give us readings to 0.01 of the pH. In a nutshell, indicators can tell us whether something is acidic or alkaline, and how acidic or alkaline it is. Red litmus paper changes color for alkalis and blue litmus paper changes color for acids. And universal indicator can give us more detail indicating where on the 0 to 14 pH scale a solution is. Acids are from 0 to 6, neutral is 7 and alkalis are 8 to 14.[6] Buffer Solutions [8] • buffers resist changes in pH, • are made up of weak acid/base and its conjugate base/acid, • buffer resist changes in pH by the weak acid consumes any hydroxide ion (-OH) added to the solution and conjugate base consumes any hydronium ion(H+) added to the solution. Microscope [9] is an item of scientific machinery that enables us to observe very small objects that are normally invisible to the naked eye. Magnification is the process of making objects appear larger using lenses like those in spectacles or a magnifying glass. Magnification also explains how much larger an object seems to be when viewed via a microscope. When anything is magnified 100 times, it appears 100 times larger than it actually is. We refer to the device we commonly use, as a light microscope. It makes use of a light to let us to see the intricate features of the object we are examining (our sample). A light microscope can be used to observe bacteria, parasites, and cells. Table 3. microscope function Eyepiece Focus wheel Light source Objective lens On/Off button Clips 3. A lens in the eyepieces enlarges the sample. You view your sample through the eyepieces. The objective lens contains a second lens that pairs up with the first to create the size of the image you see. To prevent visual blur, it raises and lowers the lenses. The distance between the lenses and the sample must be correct for an image to be in focus. The lenses distort the sample's light so that it creates a sharp image in your eye. The sample is illuminated, making it simpler to notice minute features. Magnifies the sample to make it look bigger. The eyepieces have 2 lenses. The magnification of the lenses lets you know how much larger the sample appears to be. Scanning (4x), Low (10x), High (40x), and Oil Immersion (100x). to turn the light source on and off. to hold the microscope slides that has the sample, tight on the base while you look at it. Materials Table 4. materials used in the lab Devices Chemicals Consumables Ph meter NaCl 250 ml beaker Microscope spatula Precision balance distilled water magnetic stirrer Graduated Cylinder Small piece of foil e letter printed out Microscope slides 4 MERVE EBUARRA 4. Method • Solution preparation i. turn on the precision balance, use a small piece of foil to zero the precision balance, ii. an amount of solute (NaCl) is weighed out on the precision balance and then, iii. a portion of solvent (distilled water) is added to the graduated cylinder, iv. the solute (NaCl) is added to the 250mL beaker, v. the solvent (distilled water) is added from the graduated cylinder to the 250mL beaker, vi. the magnate is added to the solution in the 250mL beaker, vii. the beaker is placed on the magnetic stirrer, after turning on the device, viii. let the mixture swirl until all of the solute is dissolved in the distilled water, ix. after that, turn off the magnetic stirrer and prepare to measure the pH. • pH x. the pH meter is turned on, xi. the electrode is removed from the tube, washed with ethanol, and placed inside the beaker, xii. wait till the pH meter stabilize to get your answer, xiii. Turn off the pH meter, wash the electrode again, and cover the electrode with the tube, again. • Microscope xiv. One microscope slide is needed, xv. the cut-out letter e is placed on the microscope slide right side faced up, xvi. a little bit of water is put on the letter (dot that covers the letter), xvii. the cover slip is placed on the letter at 45o,slowly to avoid any bubbles, it should stick, xviii. The slide is placed on the microscope using the clips properly to hold the slide, 5. xix. Turn on the microscope, xx. Eyepiece, Focus wheel, Light source and Objective lens are used, as mentioned in table3. to get the results, xxi. XY-axis can be used to move around the slide. xxii. Using a soft tissue moistened with ethanol, wipe the lenses and stage if any immersion oil got used, and the slide. Results • For the solution, we poured 150mL of distilled water in the graduated cylinder, • We measured NaCl and got 0.154g, • We added the NaCl to the 250mL beaker and we added the distilled water to them, 0.154𝑔 0.154𝑔 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝐶𝑙 = 150𝑚𝐿 × = 0.15𝐿 × = 0.0231 𝑔 𝑚𝑜𝑙𝑒 𝑁𝑎𝐶𝑙 1𝐿 1𝐿 After we waited for the solution stir well, at speed = 400, we used the pH meter to find the pH • 5 MERVE EBUARRA • The pH we got was = 7.16 Figure 3. letter e at 4x under the microscope Figure 4. letter e at 10x under the microscope Figure 5. letter e at 40x under the microscope 6. Discussion We run this experiment in order to understand how pH meter works and microscopic techniques. The way pH meter works [6] is that based on its three components that make up a pH meter, which they are an internal electrode, a reference electrode, and a high input impedance meter. The internal electrode and reference electrode are frequently present in glass probes. The reference electrode is usually built from the same materials as the internal electrode, which is a silver wire covered with silver chloride (Ag/AgCl wire). A buffer solution with a pH of 7 is located inside the probe (KCL) cause its pH neutral. The inner glass od the electrode glass membrane is in contact with KCL, and the outer glass membrane is in contact with the sample. The [H+] difference between the reference buffer inside the probe and the sample solution is the measured pH we got. Basic solution: pH = 7.16 > 7, solutions - that are basic (or alkaline)- have pH values higher than 7, meaning that hydroxide ion activity is greater than that of hydrogen ion. The color should be blue because it’s base, pH ranged between 6.9-7.6, the indicator is bromothymol blue. The number of pH we got was expected due the inconsistency of the NaCl we used, we were asked to use 0.150g, but of measured amount was 0.154g. Now for the microscope part: For fig3. • Starting at the lowest objective, we placed the slide on the stage and secured it with the clips, 6 MERVE EBUARRA • We centered the specimen so the eye can see, using the XY-axis knob, • using the focus wheel while looking through the eyepiece we started to slowly move the stage, • starting at the highest point and slowly move lower the stage until we have a clear view of the specimen, • made the resolution sharper by using the focus wheel, • we switched to the next objective once the image got focused without moving the stage, • made us see an enlarged view of the letter e, • we sharpened the image again using the focus wheel at 4x, For fig4. • once focused, again without moving the stage, we switched to objective to 10x, • at 10x, we sharpened the image again using the focus wheel, For fig5. • once focused, again without moving the stage, we switched to objective to 40x, • we needed to adjust the light using the light source knob, because the field of view got considerably darker at 40x, • at 40x objective or higher we used the focus wheel only, • at 40x, we sharpened the image again using the focus wheel only, additionally, if we were to use the objective at 100x, we should add an immersion oil midway when switching between 40x and 100x objectives, again without moving the stage. 7 MERVE EBUARRA 7. References [1] Periodic Table of the Elements. LibreTexts CHEMISTRY. Published July 6, 2022. https://chem.libretexts.org/Ancillary_Materials/Reference/Periodic_Table_of_the_Elements [2] Britannica, T. Editors of Encyclopaedia (Invalid Date). solution. Encyclopedia Britannica. https://www.britannica.com/science/solution-chemistry [3] Britannica, T. Editors of Encyclopaedia (Invalid Date). solvent. Encyclopedia Britannica. https://www.britannica.com/science/solvent-chemistry [4] Lorig, C. H. and Gruner, . Holger (Invalid Date). mineral processing, concentration. Encyclopedia Britannica. https://www.britannica.com/technology/mineral-processing [5] Halpern J, ed. Chemistry: The Central Science, 4: Reactions in Aqueous Solution, 4.5: Concentration of Solutions. LibreTexts. Published September 16, 2022. https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry__The_Central_Science_(Brown_et_al.)/04%3A_Reactions_in_Aqueous_Solution/4.05%3A_Concentrat ion_of_Solutions#title [6] Britannica, T. Editors of Encyclopaedia (Invalid Date). pH. Encyclopedia Britannica. https://www.britannica.com/science/pH [7] Helmenstine, Anne Marie, Ph.D. (2020, August 26). 10 Facts About Acids and Bases. https://www.thoughtco.com/facts-about-acids-and-bases-603669 [8] Britannica, T. Editors of Encyclopaedia (Invalid Date). buffer. Encyclopedia Britannica. https://www.britannica.com/science/buffer-chemistry [9] Ford, B. J. and Shannon, . Robert R. (Invalid Date). microscope. Encyclopedia Britannica. https://www.britannica.com/technology/microscope 8