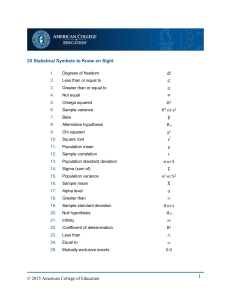
BL1401 General Biology for Majors I St. Mary’s University, Department of Biological Sciences Module 3b: What do Yeast Like to Eat? Data Analyses Learning Outcomes for Today’s Lab 1. Students will learn how to write a testable hypothesis. 2. Students will learn how to summarize data using descriptive statistics such as mean. range, standard deviation, variance. 3. Students will practice organizing and recording experimental data. 4. Students will practice calculations associated with basic descriptive statistics. 5. Students will practice choosing the best means of presenting their analyzed data (type of graph or table). 6. Students will practice explaining the relevance of their experimental data and using it to evaluate their testable hypothesis. 7. Students will identify the different types of variables and explain their role in a well-designed experiment. 8. Students will explain the importance of sample size and replication in data interpretation. 9. Students will distinguish between an absolute value and a relative value, identify examples of each from their data, and explain the value of each in communicating their results. 10. Students will explain how different carbon sources affect yeast growth. with basic descriptive statistics. Prelab Introduction and Background This week in lab, you will collect and analyze the data for the experiment you prepared in lab last week and determine what yeast preferred as a nutrient source and how well they grew on alternative carbon sources (relative to glucose). Please review previous materials, the Scientific Method, and read the background information which follows. In preparation for the lab report, you should also read Chapter 4 of A Student Handbook for Writing in Biology by Karin Knisely. A scanned copy of Chapter 4 will be available on Canvas. Elements of an Experiment (adapted from Vodopich and Moore, and https://www.canyons.edu/Faculty/medinalopezp BioSci 100 Open Educational Resource) Formulation of a Hypothesis Last week, prior to beginning the experiment, you were asked to formulate a hypothesis. This is because well-organized experiments to answer questions require that questions be restated as testable hypotheses. A hypothesis is a statement that clearly states the relationship between biological variables. A good hypothesis identifies the organism or process being investigated, identifies variables being recorded, and implies how the variables will be compared. An analysis of your experiment will determine whether you accept or reject your hypothesis. Consider your hypotheses from last week’s lab. Do they fit the criteria described above? Could your hypotheses be refined to meet these criteria? Could your hypothesis be considered a null hypothesis? A null hypothesis (H0) would be a statement that predicts the independent variable would not show a difference 1 in the dependent variable. The null hypothesis is a common way to state a clear and testable hypothesis. Well written null hypotheses are very testable. About Experiment Variables 1. Independent Variable: This is the variable that is changed by the investigator. This variable is chosen because the investigator predicts that changing it will impact the dependent variables and a functional relationship can be established. Consider what the Independent Variable was in the experiment you prepared last week. 2. Dependent Variable: This is the variable that is measured, counted or recorded by the investigator. It is the factor that varies in response to conditions manipulated with respect to the independent variable. Consider what the Dependent Variable is for your experiment that you will measure this week in lab. 3. Standardized Variables: These are the variables that are kept equal in all treatments so that any changes in the dependent variable can be attributed solely to changes in the independent variable. Consider what the Standardized Variables were for the experiment you prepared last week. 4. Experimental Treatment: The experimental treatment is the one where the independent variable is manipulated. 5. Control Treatment: A control treatment is one where the independent variable is either eliminated or set at a standard value. The results of the control treatment are compared to the results of the experimental treatment to determine if manipulating the independent variable had a measurable effect on the dependent variable. Consider what the Control was in the experiment you prepared last week and whether there could have been other types of controls. 6. Sample Size and Replication: It is important for an experiment to be repeatable; this increases our confidence that that the observed results are due to changes in the independent variable. All biological systems contain natural variability and will therefore respond in slightly different ways each time an experiment is performed. By repeating the experiment either over time (replication) or by increasing the number of organisms that are experimented on (sample size), the investigator can obtain an average value that more accurately reflects the true result. Usually, the more replications there are, the better. Why might this be? Consider the Sample Size and Replicates for this experiment; do you think they are adequate? The results of an experiment are the recorded change in the dependent variable. Data are reported in objective terms that allow for independent interpretation by anyone reading the report. The preferred method of reporting data is the presentation of results in tables and graphs that provide a quick and clear overview of any observed effects. There are two types of data: qualitative and quantitative. Types of Data: Both types of data are valid and important. In some instances, an experiment may result in just one or the other type of information. Frequently, investigators may collect and report both types of data. 2 1. Qualitative Data: These kinds of data include qualities such as color, smell and taste. These are subjectively perceived and can be difficult to express in an objective manner. While everyone conducting the experiment may agree that the solution changed color, there may be variation in what individuals identify as blue, light blue, etc. Consider what kind of qualitative data the experiment you prepared last week may generate. 2. Quantitative Data: These kinds of data include qualities that can be measured objectively such as weight, volume, length and temperature. Quantitative data have a number associated with them and can be reported in universally accepted metric units. This makes it easy for others to interpret the results. Consider what kinds of quantitative data the experiment you prepared last week may generate. Analyzing Experimental Data Analysis begins with summarizing the raw data. Typical descriptive statistics include: • The mean (x) of the response variable for replicates of each treatment and for the control replicates. The mean is a single number that represents central tendency of the response variable. • The variation within each set of replicates: o Range, which is the highest and lowest values in a set of replicates. A wide range would indicate much variation in the data. o Standard deviation, summarizes variation just as the range does, but is less affected by extreme values. Calculating the standard deviation requires you to calculate the mean, calculate the deviation of each sample from the mean, square each deviation, and then sum the deviations. The summation is the sum of squared deviations: N Sum of squared deviations = Σ (x – x) i 2 i=1 where N = total number of samples x = the sample mean xi = measurement of an individual sample N The summation sign Σ i=1 means to add up all the squared deviations from the first one (i=1) to the last one (i=N). Variance is calculated from the sum of the squared deviations by dividing it by the number of samples minus one (N-1): Variance = sum of squared deviations N ‒1 Finally, Standard Deviation (SD), is equal to the square root of the variance: SD = square root (variance) 3 SD is often reported as a ± value with the mean in a statement such as, “The mean height of the plants was 47 ± 2 in.” (47 is the mean inches, and 2 inches is the standard deviation.) Sometimes, raw data or summarizing the dependent variable values is not as informative as reporting relative values which can also be summarized by calculating the mean or standard deviation. This involves taking the dependent variable measures and converting them to values that describe how a treatment increased a biological response or changed relative to a control. Some examples of relative values are: Percent of control, Percent change, Percent increase, or Percent decrease To calculate these, you would obtain the mean of the dependent variable and then use this value to calculate percent change/increase/decrease for each of your experimental sample values. Then those calculated values would be summarized. When might relative values be more informative than absolute values? Shown below is the calculation of descriptive statistics for Absolute Values and for Relative Values with example data. Summary Statistics for Absolute Values Condition Control Control Control Drug 1 Drug 1 Drug 1 Drug 2 Drug 2 Drug 2 Replicate 1 2 3 mean 1 2 3 OD600 4.2 3.4 3.1 3.6 Deviation Deviation2 0.6 0.40 -0.2 0.03 -0.5 0.22 sum 0.65 -0.3 -0.1 0.4 mean 4.5 4.7 5.2 4.8 -0.1 0.6 -0.5 mean 2.1 2.8 1.7 2.2 1 2 3 Variance SD 0.32 0.57 sum 0.09 0.01 0.16 0.26 0.13 0.36 sum 0.01 0.36 0.25 0.62 0.31 0.56 4 When calculating the relative values in the table at right, the treatment samples were compared to the control. Therefore, in doing so, the control values represent 100% OD600 and the treatments either increase or decrease OD600. Calculation of Relative Values and Summary Statistics Condition Control Control Control Drug 1 Drug 1 Drug 1 Drug 2 Drug 2 Drug 2 Replicate 1 2 3 mean OD600 4.2 3.4 3.1 3.6 % of Control 117.8 95.3 86.9 100.0 126.2 131.8 145.8 134.6 -8.4 -2.8 11.2 mean 4.5 4.7 5.2 4.8 mean 2.1 2.8 1.7 2.2 58.9 78.5 47.7 61.7 1 2 3 1 2 3 Deviation 17.8 -4.7 -13.1 sum Deviation2 315.31 21.84 171.19 508.34 Variance SD 254.17 15.94 sum 70.75 7.86 125.78 204.38 102.19 10.11 -2.8 16.8 -14.0 sum 7.86 282.99 196.52 487.38 243.69 15.61 It is possible to use software such as Excel to calculate descriptive statistics using equations such as =sum(data cells you wish to sum) to get ∑, =average (data cells you wish to average) to obtain a mean, =sqrt(data cell you wish to get the square root of), = (data cell 1) – (data cell 2) to subtract value in one data cell from another, etc. You may even calculate standard deviation using, =std(data cells) or =stdev.s(data cells), depending on which version of Excel you have. However, it is helpful to work through the calculations manually so that you better understand the significance of variation values. Testing Hypotheses Determining whether one mean is higher than another is not an adequate test because natural variation will always make the two means at least slightly different; this might be the case even if the two independent variables have the same effect on the dependent variable. Therefore, the means and the variation about the means must be compared to determine if the means are not just different but significantly different. To be significantly different, the differences between means must be due to the independent variable and not just due to natural variation (or chance). If the difference is significant, then the null hypothesis is rejected. Testing for significant difference is typically done with statistical methods that calculate the probability that the means are significantly different. This is beyond the scope of this class. 5 A simpler method to test for significant difference between means will be employed in this class. For the purpose of this experiment, we will agree that means are significantly different from each other IF the means plus or minus one-half of the standard deviation do not overlap. Using the data from the tables above, one-half of the standard deviation was calculated, then added and subtracted from the mean. The two values for each condition were then compared with each other to see if there was any overlap. Absolute Values Condition Mean OD600 Control 3.6 Drug 1 4.8 Drug 2 2.2 SD 0.57 0.36 0.56 1/2 SD 0.285 0.18 0.28 mean + 1/2 SD 3.885 4.98 2.48 mean - 1/2 SD 3.315 4.62 1.92 Overlap? No No No Relative Values Condition Mean % of Control Control 100 Drug 1 134.6 Drug 2 61.7 SD 15.94 10.11 15.61 1/2 SD 7.97 5.055 7.805 mean + 1/2 SD 107.97 139.655 69.505 mean - 1/2 SD 92.03 129.545 53.895 Overlap? No No No For example, using the relative values, you can see that the control ranges from 92.03-107.97% which does not overlap with either Drug 1, 129.5 – 139.66%, or Drug 2, 53.9 – 69.5%. Nor do the values for Drug 1 or Drug 2 overlap. This means that, in the case of this lab, there is significant difference between the control, Drug 1, and Drug 2. More specifically, treatment with Drug 1 significantly increased OD600 approximately 35% relative to the control while Drug 2 significantly decreased OD600 by 40%. Presenting Data Refer to your writing guide for practices on analyzing and presenting data. Briefly, it is important to point out that RAW (unanalyzed or unsummarized) data should not be included in a lab report. For example, the tables shown in the previous pages would be UNACCEPTABLE. While it is tempting to show “all” of the data you have obtained and “all” of the calculations you have made—because collecting and analyzing data is a lot of work—the inclusion of these is confusing to your audience. Data should be presented in a way that is both straightforward to a reader and gives them a sense for the major trends and variation of your data. Summarized data (means per group and standard deviation or ± ½ standard deviation) in the form of a table (good) or a graph (great) are typically how data are reported in a lab report or paper. Safety Considerations • • • • Wear safety glasses. Wear closed toe shoes. Treat all yeast solutions as though they contain a pathogenic microorganism; avoid getting on you and wash your hands when you spill and prior to leaving lab. You will be working with glass (slides, coverslips, and glass tubes). Use care to avoid dropping or breaking and associated cut hazards. 6 • There are no burn hazards today, as the data collection procedure will not require you to use sterile technique. This is because the yeast you use today will not be propagated further. Activity Today is lab, you will determine the extent of yeast growth with each nutrient source. The extent of growth will be based on the OD600 of each culture. Therefore, you will be measuring and then analyzing the OD600 of each culture. Please follow the procedure below. 1. After reading the introduction to this lab, please take a moment to rewrite your hypotheses for this experiment and to also write the null hypothesis. Record in your notebook. 2. Prior to data collection, vortex cultures to mix and observe your cultures. a. Record qualitative data for all of your cultures in your notebook. b. Consider all types of qualitative data and note that microscopes are available for your use. 3. Measure the OD600 for your cultures. a. It may be necessary to dilute for some cultures to obtain OD600 values less than 2.0 and higher than 0.01. Note: • Not all cultures will need dilution. • When considering how much to dilute, think about what the culture looks like to your eye and the starting OD600 (see your notebook from last week). Think about what should happen with every doubling of yeast and the amount of time they were allowed to grow. On the shorter end, yeast can double in as little as an hour and a half. • You don’t need to make large volumes of dilutions. Remember, you only have 5 ml of cultures. b. Record the calculation for your dilution (if needed), the dilution (if applicable), the OD600 for the diluted sample (if applicable), and the dilution-corrected OD600 (if applicable) in your notebook. 4. Each student should summarize the data by manually calculating the mean, sum of squared deviations, variance, and standard deviation for the raw data (OD600) each nutrient source. Record these values in your notebook. Note: • You may consult with your lab group to do this, but each student in the class is responsible for calculating this data on their own and knowing how to do it. • Individuals (not as a group) may set up a table on Excel to do the manual calculation (as shown in the data tables on page 5) but you may not use the standard deviation equation in your analyses. • If you utilize Excel, it is your responsibility to print the table and paste it into your notebook. 5. Each student should calculate the relative data (percent of glucose control) and summarize by manually calculating the mean, sum of squared deviations, variance, and standard deviation for the relative data (percent of glucose control) for each nutrient source. Record these values in your notebook. See notes from item 4 above, as they are applicable here as well. 7 6. Non-summarized percent of glucose control values should be posted on the board in the front of the classroom. (This would be three percent values for each nutrient source.) 7. Summarize the CLASS DATA by manually calculating the mean, sum of squared deviations, variance, and standard deviation for the relative data (percent of glucose control) for each nutrient source. Record these values in your notebook. See notes from item 4 above, as they are applicable here as well. 8. Test your hypotheses by calculating ±½ SD for the percent of glucose data (you may use either your own data or the class data set, but you must justify your choice). a. Record the ±½ SD values and the percent of glucose control value ranges for each nutrient source. b. Determine if there is any overlap between nutrient sources. Record in your notebook. c. Determine if you accept the null hypothesis and other hypotheses. Record in your notebook. 9. Think about what the data tells you. Do you now know what yeast like to eat? Why or why not? Think about how your data should be represented graphically. As a group, you should prepare a single DATA FIGURE that shows “what yeast like to eat.” Your figure should be properly formatted—see examples of figures in your writing guide. This figure is your “ticket” to leave lab today. Make sure everyone has a copy of the figure because you will need it for your Reflection. LRA #1 Assignment SUBMITTED ON CANVAS by February 28, 2022 Review Chapter 4 of the Knisely writing guide, paying particular attention to the section on Results and Discussion. Also review the figure you prepared in lab. For LRA#1, you should: 1. Produce a figure from your data 2. Write a brief caption for your figure 3. Write your data results (see Knisely) 4. Write a short interpretation of the data which is represented by your figure. Your short interpretation should, at a minimum, be 6-8 sentences long. By interpretation, you should address two points: a. Objectively, what do the data “say?” Describe the data, as well as their “statistical significance.” b. In your opinion, what do the data “mean?” Tie to hypothesis and the question the experiment was trying to answer. 8