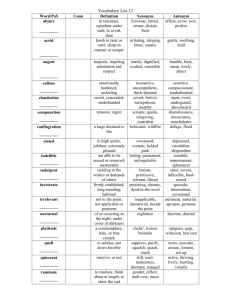
Materials Selector for Hazardous Chemicals Materials Selector for Hazardous Chemicals Michael Davies MS-6: Ammonia and Caustic Soda Publication No. MS-6 Materials Technology Institute Copyright 䉷 2004 Materials Technology Institute of the Chemical Process Industries, Inc. Printed and bound in the United States of America All rights reserved, including translations ISBN 1-57698-031-6 No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior written permission of the publisher. This document was prepared under the sponsorship of the Materials Technology Institute of the Chemical Process Industries, Inc. (MTI) and is approved for release. All data and information contained in this document are believed to be reliable; however, no warranty of any kind, express or implied, is made with respect to the data, analyses, or author of this document; and the use of any part of this document is at the user’s sole risk. MTI, the author, or any person acting on its behalf, assume no liability and expressly disclaim liability, including without limitation liability for negligence, resulting from the use or publication of the information contained in this document or warrant that such use or publication will be free from privately owned rights. Published by Materials Technology Institute www.mti-global.org Contents List of Figures .....................................................................................................xii List of Tables .......................................................................................................xv Foreword............................................................................................................ xix Section I: Ammonia Chapter 1: Introduction .....................................................................3 Chapter 2: Properties of Ammonia ..................................................7 Physical Properties ..........................................................................................8 Chemical Properties ........................................................................................9 Safety and Health Considerations....................................................................9 First Aid Procedures................................................................................ 10 Recommended Protective Equipment ...................................................... 10 Disposal, Spill, or Leak Procedures.......................................................... 11 Fire.......................................................................................................... 11 Chapter 3: Production of Ammonia ..............................................13 Chapter 4: Corrosion by Ammonia................................................19 v vi Materials Selector for Hazardous Chemicals Passivity........................................................................................................ 19 Forms of Corrosion........................................................................................ 20 General Corrosion ................................................................................... 20 Pitting Corrosion ..................................................................................... 20 Crevice Corrosion.................................................................................... 21 Intergranular Attack ................................................................................ 21 Erosion-Corrosion.................................................................................... 21 Stress Corrosion Cracking........................................................................ 21 Vapor-Phase Attack ................................................................................. 21 High-Temperature Corrosion ................................................................... 22 Dealloying............................................................................................... 22 Chapter 5: Corrosion of Metals and Alloys.................................23 Aluminum and Its Alloys .............................................................................. 23 Iron and Steel ................................................................................................ 24 Cast Irons ................................................................................................ 24 Carbon Steels........................................................................................... 25 Stress Corrosion Cracking of Steels.................................................... 26 Nitriding of Steels ............................................................................. 27 Hydrogen Attack............................................................................... 27 Temper Embrittlement ....................................................................... 29 Hydrogen Sulfide Attack ................................................................... 29 Alloy Steels ............................................................................................. 30 Chromium-Molybdenum Steels ......................................................... 30 Nickel Alloy Steels ............................................................................ 30 Stainless Steels............................................................................................... 30 Ferritic Grades......................................................................................... 31 Precipitation-Hardening Grades .............................................................. 31 Duplex Stainless Steels ............................................................................ 31 Austenitic Stainless Steels........................................................................ 32 Cast Stainless Steels................................................................................. 33 Alloys for Use at Elevated Temperatures ....................................................... 34 Creep Resistance...................................................................................... 35 Metal Dusting.......................................................................................... 35 Nickel and Its Alloys ..................................................................................... 37 Copper and Its Alloys ................................................................................... 40 MS-6: Ammonia and Caustic Soda vii Titanium and Its Alloys ................................................................................. 42 Zirconium and Its Alloys............................................................................... 43 Niobium........................................................................................................ 43 Tantalum ....................................................................................................... 43 Other Metals and Alloys................................................................................ 43 Chapter 6: Resistance of Nonmetallic Materials ........................47 Elastomers..................................................................................................... 47 Plastics .......................................................................................................... 47 Thermoplastics ........................................................................................ 48 Thermoset Resins .................................................................................... 49 Carbon and Graphite..................................................................................... 52 Ceramic Materials ......................................................................................... 52 Chapter 7: Corrosion in Contaminated Ammonia......................55 Ammonium Chloride .................................................................................... 55 Carbon Dioxide—Carbamates ....................................................................... 55 Chlorides....................................................................................................... 57 Chapter 8: Specific Production Equipment...................................59 Production Stages.......................................................................................... 59 Desulfurization Section............................................................................ 59 Primary Reformer.................................................................................... 60 Secondary Reformer ................................................................................ 63 High-Temperature Converter................................................................... 64 Carbon Dioxide Removal System ............................................................ 64 Waste Heat Recovery System................................................................... 64 Ammonia Synthesis................................................................................. 64 Distillation Columns................................................................................ 65 Heat Exchangers...................................................................................... 65 Heaters .............................................................................................. 65 Coolers and Condensers .................................................................... 66 viii Materials Selector for Hazardous Chemicals Storage Tanks .......................................................................................... 66 Refrigerated Storage Vessels .............................................................. 66 Pressurized Storage Vessels ............................................................... 67 Piping...................................................................................................... 68 Pumps ..................................................................................................... 68 Compressors............................................................................................ 68 Valves...................................................................................................... 69 Gaskets, Seals, O-Rings, and Hoses ......................................................... 69 Bolting..................................................................................................... 70 Transportation Equipment ....................................................................... 70 Rail Car Transport (Tank Cars) .......................................................... 71 Tank Trucks ....................................................................................... 72 Marine Transport............................................................................... 72 Pipelines............................................................................................ 72 Section II: Caustic Soda Chapter 9: Introduction ...................................................................77 Chapter 10: Properties of Caustic Soda........................................81 Physical Properties ........................................................................................ 81 Chemical Properties ...................................................................................... 82 Safety and Health Considerations.................................................................. 84 Recommended Protective Equipment ...................................................... 84 Fire and Explosion................................................................................... 84 First Aid .................................................................................................. 85 Disposal, Spill, or Leak Procedures.......................................................... 85 Caustic Dilution ...................................................................................... 85 Chapter 11: Production of Caustic Soda ......................................87 Production Processes ..................................................................................... 88 Diaphragm Cell Process .......................................................................... 88 Mercury Cell Process ............................................................................... 89 Membrane Cell Process ........................................................................... 89 Concentrated Solutions and Solid Caustic Soda ....................................... 90 Impurities...................................................................................................... 90 MS-6: Ammonia and Caustic Soda ix Chapter 12: Corrosion by Caustic Soda........................................93 Passivity........................................................................................................ 93 Forms of Corrosion........................................................................................ 94 General Corrosion ................................................................................... 94 Localized Corrosion................................................................................. 95 Galvanic Corrosion.................................................................................. 95 Erosion-Corrosion.................................................................................... 95 Intergranular Attack ................................................................................ 95 Dealloying............................................................................................... 95 Liquid Metal Embrittlement .................................................................... 96 Stress Corrosion Cracking........................................................................ 96 High-Temperature Corrosion ................................................................... 96 Chapter 13: Corrosion of Metals and Alloys...............................99 Aluminum and Its Alloys .............................................................................. 99 Iron and Steel .............................................................................................. 100 Cast Irons .............................................................................................. 100 Carbon and Low-Alloy Steels ................................................................ 101 Stainless Steels............................................................................................. 106 Ferritic Grades....................................................................................... 106 Precipitation-Hardening Grades ............................................................ 109 Duplex Grades ...................................................................................... 109 Austenitic Grades .................................................................................. 111 Cast Stainless Steels............................................................................... 113 High-Performance Austenitic Alloys ........................................................... 115 Nickel and Its Alloys ................................................................................... 121 Chromium-Free Alloys .......................................................................... 122 Chromium-Bearing Alloys ..................................................................... 126 Copper and Its Alloys ................................................................................. 128 Titanium and Its Alloys ............................................................................... 129 Zirconium and Its Alloys............................................................................. 130 Other Nonferrous Metals and Alloys........................................................... 131 x Materials Selector for Hazardous Chemicals Chapter 14: Resistance of Nonmetallic Materials ....................137 Elastomers................................................................................................... 137 Plastics ........................................................................................................ 138 Thermoplastics ...................................................................................... 138 Thermoset Resin Materials .................................................................... 140 Carbon and Graphite................................................................................... 141 Ceramics ..................................................................................................... 142 Chapter 15: Corrosion in Contaminated Caustic and Mixtures......................................................................145 Contaminants in Caustic Soda ..................................................................... 145 Chlorates ............................................................................................... 146 Chlorides............................................................................................... 148 Chlorine/Hypochlorite.......................................................................... 149 Mercury................................................................................................. 149 Sulfur .................................................................................................... 150 Iron ....................................................................................................... 150 Sodium Hydroxide Treatments .................................................................... 151 Petroleum Refining................................................................................ 151 Bauxite Refining .................................................................................... 151 Soap Manufacture ................................................................................. 152 Sodium Hydrosulfide Production .......................................................... 152 Caustic Fusion Reactions ....................................................................... 152 Metal Finishing ..................................................................................... 153 Pulp and Paper...................................................................................... 153 Caustic Contamination ................................................................................ 154 Contamination of Steam ........................................................................ 154 Contamination of Organic Media .......................................................... 155 Contamination of Molten Sodium.......................................................... 155 Chapter 16: Related Chemicals ....................................................159 Soda Ash..................................................................................................... 159 Potassium Hydroxide .................................................................................. 160 MS-6: Ammonia and Caustic Soda xi Chapter 17: Summary of Corrosion of Materials in Caustic Soda.............................................................................163 Chapter 18: Specific Production Equipment...............................167 Production Equipment ................................................................................ 167 Pressure Vessels..................................................................................... 167 Brine Circulation Piping ........................................................................ 169 Evaporators and Crystallizers................................................................ 169 Salt Separators....................................................................................... 170 Caustic Soda Handling ................................................................................ 170 Heat Exchangers.................................................................................... 171 Heaters ............................................................................................ 171 Coolers ............................................................................................ 171 Storage Tanks ........................................................................................ 171 Piping.................................................................................................... 173 Pumps ................................................................................................... 175 Valves.................................................................................................... 175 Gaskets, Seals, and O-Rings................................................................... 176 Shipping of Caustic Soda............................................................................. 177 Appendix A. Nominal Composition of Alloys...........................181 Appendix B. Approximate Equivalent Grade of Some Cast and Wrought Alloys ........................................................185 Appendix C. Glossary of Corrosion and Materials Terms ......187 Appendix D. Glossary of Acronyms and Abbreviations..........191 Index ..................................................................................................193 List of Figures Section I—Ammonia Figure 3.1 Figure 3.2 Figure 3.3 Figure 5.1 Figure 5.2 Figure 5.3 Figure 5.4 Figure 5.5 Figure 6.1 Figure 8.1 Figure 8.2 Figure 8.3 Figure 8.4 General View of Ammonia Production Plant. (Photo courtesy of Sasol Ltd, Secunda, RSA). Block Diagram of the Steam/Air Reforming Process for Ammonia Production. Block Diagram of the Partial Oxidation Process for Ammonia Production. Operating Limits for Steels in Hydrogen Service, API 941. Metal Wastage Rates of Nickel Alloys in a Strongly Carburizing Atmosphere at Elevated Temperature. Corrosion of Various Copper Alloys in Deaerated Ammonia. Corrosion of Various Copper Alloys in Aerated Ammonia. The Effect of Grain Size on the Time to Cracking of Yellow Brass (C26800) in Ammonia. Wastage Rates (in mm/y) of Glass-Lined Steel in Ammonia. Vol: Surface Area ⳱ 20. Part of a Modern Ammonia Plant Using Coal as a Feedstock. (Photo courtesy of Sasol Ltd, Secunda, RSA). Ammonia Production Flow Sheet Showing Principal Items of Equipment. (Courtesy M. P. Sukumaran Nair, Fact Ltd, Cochin, India). Effect on Service Life of Overheating an HK 40 Tube Above the Prescribed Base Temperature. Stress to Rupture Data for Hp Modified Alloys Compared with HK 40. Section Ii—Caustic Soda Figure 9.1 Figure 10.1 Figure 11.1 Figure 11.2 Figure 11.3 Chlor-Alkali Plant in Australia. (Courtesy of CHEMETICS—A division of Aker Kvaerner Canada Inc.). Range of Boiling and Freezing Temperatures of NaOH. Schematic View of Membrane Cell Showing Inputs and Outputs. Block Diagram of the Production of Caustic Soda from the Various Types of Electrolytic Cells. Flow Diagram of Triple-Effect Caustic Soda Evaporator. xiii xiv Figure 13.1 Figure 13.2 Figure 13.3 Figure 13.4 Figure 13.5 Figure 13.6 Figure 13.7 Figure 13.8 Figure 13.9 Figure 13.10 Figure 13.11 Figure 13.12 Figure 13.13 Figure 13.14 Figure 13.15 Figure 13.16 Figure 13.17 Figure 13.18 Figure 14.1 Figure 14.2 Figure 15.1 Figure 15.2 Figure 17.1 Figure 17.2 Figure 17.3 Materials Selector for Hazardous Chemicals Effect of pH on the Corrosion Rate of Aluminum in NaOH at 30⬚C (left) and 60⬚C (right). Corrosion Rates of Gray Cast Iron Compared with NiResist威 in Caustic Soda. Temperature and Concentration of Caustic Soda that can Cause SCC of Carbon Steels. Temperature and Concentrations of Caustic Soda that Require Stress Relief to Prevent SCC of Carbon Steel. Corrosion Resistance of Duplex Stainless Steels in Boiling NaOH Solutions. Isocorrosion Curves (in mpy) for 304 and 316 Stainless Steels in Caustic Soda also Showing Limits of SCC. Isocorrosion Curve for a Corrosion Rate of 0.1 mm/y for 904 L (N80904), other Stainless Steels, and Titanium. Isocorrosion Curves for CF 8 (J92600) in NaOH at Equilibrium Pressure (left) and Atmospheric Pressure (right). Effect of Molybdenum and Nickel in Various Alloys on Corrosion Rate in NaOH Solutions. Effect of Nickel Content on Corrosion in NaOH of Various Alloys With and Without Oxygen in the Atmosphere. Effect of Molybdenum, Nickel, and Chromium on the Threshold Temperature at Which the Corrosion Rate Exceeds 5 mpy (0.127 mm/y) in 50% NaOH. Isocorrosion Data for Cn 7M (J92700) Compared with CD 4MCu (J93370) in NaOH Solutions. Isocorrosion Curves for CN 7M (J92700) in NaOH at Equilibrium Pressure (left) and Atmospheric Pressure (right). Isocorrosion Curves (in Mm/y) for Alloy 28 (N08028) and Standard Austenitic Stainless Steels in NaOH Solutions. The Effect of Nickel on The Corrosion of Iron-Nickel-Chromium Alloys in Caustic Soda. SCC Regions for Nickel and Other Alloys in NaOH. Isocorrosion Curve (in mpy) for Alloy 200 (N02200) and Alloy 201 (N02201) in Caustic Soda. Isocorrosion Curves for Alloy 600 (N06600) and Alloy 201 (N02201) in Caustic Soda. Wastage Rates (in mm/y) of Glass-Lined Steel in Caustic Soda. Vol: Surface Area ⳱ 20. Effect of Temperature on the Corrosion of Borosilicate Glass in 50% NaOH. Effect of Chlorates on Various Alloys in 50% Caustic at 150⬚C (302⬚F). Effect of Nickel Content on Corrosion of Various Materials in Simulated First-Effect Liquor at 185⬚C (365⬚F). Isocorrosion Curve (at 1 mpy) for Stainless Steel and Nickel in Caustic Soda. Summary Curves for SCC Regions for Carbon Steel, Stainless Steel, Nickel-Rich and Nickel-Based Alloys. Range of Use of Various Nickel Alloys in Caustic Soda. List of Tables Section I—Ammonia Table 1.1 Table 2.1 Table 2.2 Table 2.3 Table 2.4 Table 2.5 Table 5.1 Table 5.2 Table 5.3 Table 5.4 Table 5.5 Table 5.6 Table 5.7 Table 5.8 Table 5.9 Table 5.10 Table 6.1 Industrial Uses of Ammonia. Commercial Grades of Anhydrous Ammonia. Grades of Commercial Ammonia Solutions. Typical Properties of Anhydrous Ammonia. Typical Properties of Nominally 30% Aqua Ammonia. Human Physiological Response to Ammonia in Air. Corrosion Rates of Aluminum in Ammonium Hydroxide Solutions at 20⬚C (68⬚F). Corrosion Data for NiResist威 and Cast Iron in Ammonia Solutions and Environments. Ammonia SCC Mitigation Measures. Chromium Equivalents for Alloys Commonly Used in Ammonia Plants. Nitrogen Absorption in Nickel Alloys Exposed to Flowing Ammonia at 1200⬚F (648⬚C). Effect of Temperature on Nitriding Depth in Various Nickel Alloys. Nitriding Tests in an Ammonia Converter. Corrosion of Alloy 200 in 1 N Ammonium Hydroxide (1.7% NH3). Corrosion of Alloy 200 in Agitated Ammonium Hydroxide Solutions at Room Temperature. Corrosion of Alloy 400 in Agitated Ammonium Hydroxide Solutions at Room Temperature. Estimated Temperature Limits (⬚C [⬚F]) for Various Elastomers That May Be Suitable in Ammonia. xv xvi Table 6.2 Table 6.3 Table 6.4 Table 6.5 Table 6.6 Table 8.1 Table 8.2 Materials Selector for Hazardous Chemicals Suggested Temperature Limits (⬚C [⬚F]) for Various Plastics in Ammonia. Recommended Temperature Limits for Plastic-Lined Pipe in Ammonia. Suggested Temperature Limits (⬚C [⬚F]) for Various Plastics in Dual Laminate Construction in Ammonia. Suggested Temperature Limits for FRP in Ammonia Service. Estimated Temperature Limits (⬚F [⬚C]) of Commercial FRP Piping. O-Ring Materials Compatible with Ammonia and Ammonium Hydroxide. DOT Classifications for Ammonia. Section II—Caustic Soda Table 9.1 Table 10.1 Table 10.2 Table 10.3 Table 13.1 Table 13.2 Table 13.3 Table 13.4 Table 13.5 Table 13.6 Table 13.7 Table 13.8 Table 13.9 Table 13.10 Table 13.11 Table 13.12 Table 13.13 Table 13.14 Table 13.15 Table 13.16 Table 14.1 Table 14.2 Typical Uses of Caustic Soda by Industry. Physical Constants of Sodium Hydroxide (NaOH). Boiling Points of Strong NaOH Solutions. Hydrogen Ion Concentration of Various-Strength Caustic Soda Solutions at 25⬚C (77⬚F). Effect of Nickel Additions on Corrosion of Cast Irons in Boiling 50% to 65% NaOH. Corrosion Data for NiResist威 and Cast Iron in Caustic Soda. Corrosion of Cast Irons by Molten NaOH at 510⬚C (950⬚F). Corrosion of Steel by Sodium Hydroxide Solutions. Corrosion of Stainless Steels in NaOH Solutions. Corrosion Rates of Stainless Steels in 50% NaOH at 140⬚C (290⬚F). Corrosion Rates (mpy [mm/y]) in Boiling 50% NaOH Solution. Corrosion Rates of 17-4PH (S17400) in Hot, Concentrated Caustic. Lowest Temperature at Which Corrosion Rate Exceeds 5 mpy (0.127 mm/y). Corrosion Rates (mpy [mm/y]) for Various Alloys in Boiling 50% Sodium Hydroxide. Corrosion Rates (mm/y [mpy]) of Various Alloys in NaOH and NaOH/NaCl. Corrosion Rates (mm/y [mpy]) of Stainless Steels in Sodium Hydroxide at Various Conditions. Static Corrosion Rates (mpy [mm/y]) in Molten Caustic Soda. Corrosion Rates (mpy [mm/y]) of Nickel and Other Materials in Caustic Evaporation Plants. Corrosion Rates of Commercially Pure Titanium in Various Solutions of NaOH. Corrosion Rates of Zirconium (R60702) in Sodium Hydroxide Solutions and Mixtures. Dry, Hot Air Temperature Limits for Various Elastomers. Estimated Elastomer’s Maximum Temperature (⬚C [⬚F]) in VariousStrength Solutions of NaOH. MS-6: Ammonia and Caustic Soda Table 14.3 Table 14.4 Table 14.5 Table 14.6 Table 14.7 Table 14.8 Table 15.1 Table 15.2 Table 15.3 Table 15.4 Table 16.1 Table 16.2 Table 17.1 Table 18.1 Table 18.2 xvii Thermoplastics Maximum Temperature (⬚C [⬚F]) in Various Strengths of NaOH. Temperature Limits for Plastic-Lined Pipe in Caustic Soda. Temperature Limits (⬚C [⬚F]) for Various Plastics in Dual Laminate Construction in Up to 10% Caustic Soda Solutions. Thermosets as FRP Maximum Temperature (⬚C [⬚F]) in Various Strengths of NaOH. Corrosion Rate in mm/y of Borosilicate Glass in NaOH at Various Temperatures. Corrosive Weight Loss (mg/cm2/y) of Various Ceramics in 50% NaOH at 100⬚C (212⬚F). Resistance of E-Brite威 Alloy to Caustic Solutions Containing NaCl and NaClO3. Corrosion Rates (mm/y) of Various Alloys in Hot Caustic Solutions. Corrosion Rates (mm/y) of S32906 and N02200 in Boiling NaOH Solutions Simulating Membrane and Diaphragm Cell Liquors. Corrosion Rates (mm/y) of Various Alloys in NaOH/NaOCl Exposed to Liquid and Vapor. Laboratory Corrosion Tests in Potassium Hydroxide. Corrosion Rate (mm/y [mpy]) of Metals and Alloys in KOH Solutions. Alloys for Caustic Soda Service by Corrosion Resistance Category. Nickel Alloys Used in Caustic Soda Production. DOT Classifications for Caustic Soda. Foreword The original basis for the first part of this monograph on materials selection for and corrosion in the manufacture, handling, and utilization of ammonia was the NACE/ MTI Materials Selection Advisor (MSA) “CHEMCOR 12,” prepared by Michael J. Conley of Det Norske Veritas Industry, Inc., Houston, Texas. The basis for the second part on materials selection for and corrosion in the manufacture, handling, and utilization of caustic soda was the NACE/MTI Materials Selection Advisor (MSA) “CHEMCOR 6,” prepared by Dr. Peter Elliott of Corrosion & Materials Consultancy Inc., Colts Neck, New Jersey. A first draft of both sections was prepared and edited by C.P. Dillon and W.I. Pollock but was not published. Both sections have now been completely reviewed, updated, rewritten, and combined into this one volume by M. Davies of CARIAD Consultants. Most of the figures were prepared by P.J.B. Scott, also of CARIAD Consultants. Technical input and comments have been provided by a number of people, including Jim Alexander (DuPont Dow Elastomer), Daniel Leander (Sandvik), Jim McCoy (Special Metals), Sasol, and Doug Shaw (Chemetics). Information is provided in this monograph on the properties of ammonia and caustic soda, production methods, health and safety issues, forms of corrosion specific to these chemicals, definitions, and relevant specifications for materials of construction as well as supporting laboratory and field corrosion data. Alloys are identified by their UNS number together with generic or trade name where appropriate. The materials of construction preferred for manufacturing, storing, transporting, and handling ammonia, aqueous ammonia solutions, and caustic soda as commercial products are described in detail. xix Section I Ammonia 1 Introduction Ammonia is one of the most important industrial and naturally occurring chemicals in the world. It is an important link in the chain of nitrogen fixation whereby atmospheric nitrogen is converted for use by living organisms. It is the fourth most widely produced industrial chemical in the United States (after sulfuric acid, nitrogen, and ethylene). Production statistics for the United States in 1996 were 13,800, imports were 3,500, and exports were 500, all in thousand metric tons of nitrogen. The world total production of ammonia for the same year was 93,500 thousand metric tons of nitrogen. Approximately 80% of the U.S. apparent domestic ammonia consumption was for fertilizer use, including anhydrous ammonia for direct application, urea, ammonium nitrate, ammonium phosphates, and other nitrogen compounds. Ammonia was also used to produce plastics, synthetic fibers, and resins, 10%; explosives, 4%; and numerous other chemicals, 6%.1 Although ammonia’s primary use is in fertilizers, it has many other uses as shown in Table 1.1.2,3 The use of ammonia as a refrigerant to replace banned CFCs in, for example, air conditioning and household refrigerators is being actively explored.4 There is also a growing tendency to use ammonia in large, centralized applications like district cooling and heating, including heat pump applications. There is scope to introduce ammonia into the automotive industry since flammable or toxic products cannot be used in direct cooling systems for safety reasons. The use of ammonia in commercial refrigeration and in medium-size air-conditioning systems is likely to increase.5 Ammonium hydroxide, the monohydrate of ammonia, is widely used for injection into combustion air to reduce NOx emissions. It is also used instead of anhydrous ammonia in many of the applications listed previously, partly to avoid the regulatory threshold limits placed on anhydrous ammonia as an extremely hazardous substance.6 References 1. G.A. Rabchevsky, “Nitrogen (Fixed)—Ammonia,” U.S. Geological Survey, Mineral Commodity Summaries (1997), http://minerals.usgs.gov/minerals/pubs/ commodity/nitrogen/480397.pdf. 3 4 Materials Selector for Hazardous Chemicals Table 1.1 Industrial Uses of Ammonia Industry Agriculture Fertilizer Metal treating Chemical process Petroleum Mining Water and wastewater Pollution control Photochemical Petrochemical and cold storage Rubber Pulp and paper Food and beverage Fuel cells Leather Household Uses of Ammonia For direct application to crops; to provide protein for ruminating animals; as a preharvest cotton defoliant; an antifungal agent on some fruits; as a preservative for the storage of high-moisture corn To produce ammonium nitrate, urea, ammonium hydroxide, ammonium, and nitrate salts In nitriding, carbonitriding, bright annealing, furnace brazing, sintering, sodium hydride descaling, and atomic hydrogen welding In the manufacture of nitric acid; certain alkalis such as soda ash; dyes; pharmaceuticals such as sulfa drugs, vitamins, and cosmetics; synthetic textile fibers such as nylon, rayon, and acrylics; and plastics such as phenolics and polyurethanes In neutralizing the acid constituents of crude oil and for protection of equipment from corrosion For extracting metals such as copper and molybdenum from their ores To control pH; in solution form to regenerate weak anion exchange resins; with chlorine to produce potable water; as an oxygen scavenger in boiler water treatment In stack emission control systems to neutralize sulfur oxides from combustion of sulfur-containing fuels; to control NOx in both catalytic and noncatalytic applications; to enhance the efficiency of electrostatic precipitators for particulate control As the developing agent in white printing and blue printing and in the diazo duplication process In industrial refrigeration systems For the stabilization of natural and synthetic latex to prevent premature coagulation For pulping wood; as a casein dispersant in the coating of paper In industrial refrigeration systems; as a source of nitrogen for yeast and microorganisms; in the hydrogenation of fats and oils As a source of hydrogen As a curing agent; a slime and mold preventative in tanning liquors; as a protective agent for leathers and furs in storage As commercial and household cleaners and detergents MS-6: Ammonia and Caustic Soda 5 2. Anon, “Ammonia (Anhydrous) Chemical Backgrounder,” NSC, National Safety Council (2003), http://www.nsc.org/library/chemical/ammonia.htm. 3. Anon, “Uses of Ammonia” (Mt. Laurel, NJ: RM Technologies Inc., 2003), http://www.rmtech.net/uses_of_ammonia.htm. 4. Anon, “Two Japanese Companies Team Up to Provide CFC Alternatives,” zonAction, 41 (April 2002): p. 3, http://www.uneptie.org/ozonaction/library/ oan/oan41/oan41e.pdf. 5. P. de Larminat, “Expanding the Use of Ammonia,” ASHRAE Journal 42, 3 (2000): pp. 35–40. 6. Anon, “Aqua Ammonia Properties” (Mt. Laurel, NJ: RM Technologies Inc., 2003), http://www.rmtech.net/Aqua%20Ammonia.htm. 2 Properties of Ammonia Ammonia (CAS 7664-41-7), also known as liquid ammonia or anhydrous ammonia, is a colorless gas with a pungent, suffocating odor. It is a major raw material for many nitrogen-bearing compounds and as a constituent in processes other than its own manufacture (Chapter 3). It can develop characteristics related to the nature and degree of contamination by other chemicals (Chapter 7). Pure, anhydrous ammonia is a colorless, pungent, suffocating gas comprising three atoms of hydrogen and one atom of nitrogen (NH3). Its odor is familiar from the use of “household ammonia,” a dilute aqueous solution, or other cleaners containing low levels of ammonia. It is also the smell in smelling salts, sometimes called ammonia but are, in fact, ammonium carbonate crystals. Ammonia liquefies readily under pressure at room temperature and is usually stored and shipped in the liquid state. A relatively stable compound, NH3 requires a high heat source for ignition in air. Even in the presence of a suitable ignition source, there is a relatively narrow flammable concentration range (16%–25% by volume in air). Consequently, conditions favorable for ignition in air are seldom encountered in normal operation and handling. However, care must be taken with ammonia/air mixtures in confined spaces. Anhydrous ammonia is available in a number of different commercial grades as shown in Table 2.1.1 Ammonium hydroxide (CAS 1336-21-6), the monohydrate of ammonia, is a weak base or alkali. It is an excellent acid neutralizer. It also comes in different commercial strengths and grades, some of which are shown in Table 2.2.1 Ammonium hydroxide is also known as ammonia solution, aqueous or aqua ammonia, ammonia water, or ammonia monohydrate. It has a boiling point of Table 2.1 Commercial Grades of Anhydrous Ammonia Property Commercial Refrigeration Metallurgical Water content Oil content ⬍5,000 ppm ⬍5 ppm 75 ppm max 4 ppm max 33 ppm max 2 ppm max 7 8 Materials Selector for Hazardous Chemicals Table 2.2 Grades of Commercial Ammonia Solutions Constituent Ammonia as NH3 Chlorides Carbonate as CO2 Appearance Nitrogen content Commercial Agricultural 19%–29% (Ⳳ 0.5%) ⬍1.0 ppm ⬍1.0 ppm Clear 15.6%–24% 24.5%–25.3% N/A N/A Clear 20.0%–20.8% ⳮ77⬚C (ⳮ107⬚F) and is soluble in water at ambient temperatures to about 90 parts in 100 (@47%). Ammonium hydroxide is corrosive to aluminum, copper, lead, nickel, silver, tin, zinc, and various alloys of these metals and galvanized surfaces. Physical Properties The physical properties of anhydrous ammonia are shown in Table 2.3.1,2,3 Similar properties are given for commercial ammonium hydroxide solution in Table 2.4.5,6 Table 2.3 Typical Properties of Anhydrous Ammonia Property Molecular weight Boiling point at 1 atm Freezing point at 1 atm Critical temperature* Critical pressure* Latent heat of evaporation Relative density vs. air Vapor density at 1 atm, ⳮ33.3⬚C (ⳮ28⬚F) Liquid density at ⳮ33.3⬚C (ⳮ28⬚F) Specific volume at 1 atm, ⬃ 0⬚C (32⬚F) Autoignition temp. (iron catalyst) Autoignition temp. (quartz container) Specific heat at 1 atm, 25⬚C (77⬚F) Constant pressure (CP) Constant volume (CV) Vapor pressure: at 25.7⬚C (78.3⬚F) at ⳮ77.7⬚C (ⳮ107.9⬚F) Data 17.03 ⳮ33.4⬚C (ⳮ28.17⬚F) –77.7⬚C (ⳮ107.9⬚F) 113⬚C (270.32⬚F) 112.5 atm (11.4 MPa) 1.37 kJ/g (588.7 Btu/lb) 0.597 0.888 kg/m3 (0.055 lb/ft3) 682 kg/m3 (42.6 lb/ft3) 1.297 m3/kg (20.78 ft3/lb) 651⬚C (1,204⬚F) 850⬚C (1,562⬚F) 35.652 K/mol 2.189 kJ/kg ⬚C (0.5232 Btu/lb ⬚F) 1.672 kJ/kg ⬚C (0.3995 Btu/lb ⬚F) 1,013 kPa (10 atm) 6.077 kPa (0.067 atm) *Critical temperature is the temperature above which a given gas cannot be liquefied. Critical pressure is the pressure at which a gas may just be liquefied at its critical temperature.4 MS-6: Ammonia and Caustic Soda 9 Table 2.4 Typical Properties of Nominally 30% Aqua Ammonia Property Molecular weight Boiling point Melting/freezing point Vapor pressure at 20⬚C (68⬚ F) Specific gravity at 15⬚C (59⬚ F) Vapor density (air ⳱ 1) at 15⬚ C (at 0⬚C, 101.3 kPa) Liquid density (at 0⬚C, 101.3 kPa) Bulk density Solubility Data 35.06 27.2⬚ C (81⬚F) ⳮ72.4⬚C (ⳮ98.3⬚ F) 475 mm Hg (162 kPa) 0.895 g/ml 0.618 0.7714 g/L 0.6386 g/cm3 896 kg/m3 Completely soluble in water Chemical Properties Ammonia is alkaline and caustic. A 17.03-g/L solution (1 N) has a pH of 11.6; a 1.7g/L solution (0.1 N) has a pH of 11.1, and a 0.17-g/L solution (0.01 N) has a pH of 10.6. A 5% solution has a pH of 12.2, a 10% solution is pH 12.4, and at 30% the pH is 13.5. It is highly soluble in water (529 g/L at 20⬚C) and soluble in alcohol, chloroform, and ether. It is easily liquefied under pressure. It is incompatible or reactive with strong oxidizers, acids, halogens, and salts of silver and zinc. It is corrosive to copper and galvanized steel; liquid ammonia will attack some plastics, rubber, and coatings.7 Materials that should not be brought into contact with ammonia or ammonium hydroxide include the following:6 • Oxidizing agents (e.g. perchlorates, chlorates, hydrogen peroxide, chromic trioxide, nitrogen oxides, calcium, or sodium hypochlorite)—can react violently or explosively. • Heavy metals and their salts (e.g. silver, gold, lead, mercury, or zinc, especially halide salts)—may form shock-sensitive compounds that may explode when dry. • Halogens (e.g. chlorine, bromine, fluorine, or iodine) or interhalogens (e.g. bromine pentafluoride, or chlorine trifluoride)—can react violently or form explosive chemicals. • Nitromethane—increases the sensitivity of nitromethane to detonation; forms salts, which are explosive when dry. • Acids, acid anhydrides, and acid chlorides—can react violently or explosively. • Calcium—reacts with evolution of heat; may ignite at higher temperatures. • Acrolein, propiolactone, and propylene oxide—mixing with 28% ammonium hydroxide in a closed container caused a violent reaction. Safety and Health Considerations Ammonia is a naturally occurring chemical in all mammalian species, with a normal blood level of about 1.0 mg/liter. Because it is highly water soluble, ammonia vapors 10 Materials Selector for Hazardous Chemicals irritate and can damage eyes and mucous membranes of the nose, throat, and lungs. At higher concentrations, ammonia is corrosive to tissue and exposure can be fatal. Anhydrous liquid ammonia produces second-degree burns on the skin and extensive destruction of the anterior chamber in the eye. Liquid ammonia can freeze the surface of the skin, causing thrombosis of surface vessels, ischemia, and necrosis.7 The human response to various concentrations of ammonia in air is shown in Table 2.5.1,8 This table also shows the current limits set by various regulatory bodies to protect humans that might come into contact with ammonia. First Aid Procedures The following specific steps are recommended to alleviate the results of physical contact with ammonia: • Eyes—Flush with copious quantities of water for at least 15 minutes. If irritation persists, obtain medical attention. • Skin—Wash off with water. If irritation persists, obtain medical attention. • Inhalation—Remove from exposure. If breathing is difficult or discomfort persists, obtain medical attention. • Ingestion—Rinse mouth with water. Give copious amounts of water to cause dilution in stomach. Do not induce vomiting. Recommended Protective Equipment A NIOSH/MSHA acid-gas respirator with full face piece or, in other severe exposures, a self-contained breathing apparatus is recommended. Eyes should be pro- Table 2.5 Human Physiological Response to Ammonia in Air Vapor Concentration (ppm) 5 25 35 50 300 400–700 1,700 2,000–5,000 5,000–10,000 Limit or General Effect Odor detectable by most persons NIOSH/ ACGIH REL ACGIH STEL OSHA PEL NIOSH IDLH Immediate nose and throat irritation Severe coughing; severe eye, nose, and throat irritation Severe coughing; severe eye, nose, and throat irritation Respiratory spasms; rapid asphyxia Exposure Period Unlimited 8-hour TWA 15-minute TWA 8-hour TWA — 30 minutes to 1 hour causes serious effect Could be fatal after 30 minutes Could be fatal after 15 minutes Fatal within minutes MS-6: Ammonia and Caustic Soda 11 tected by chemical goggles. Rubber, neoprene, or other resistant elastomer gloves should be used when in contact with ammonia. A rubber apron and boots are suggested, especially in release situations. When storing or handling ammonia goggles and/or face shields, rubber aprons, gloves, and boots should be worn. When unloading bulk vehicles, personnel should wear chemical goggles and rubber or neoprene gloves. All fittings should be properly secured prior to energizing the unloading system. Care should be taken to avoid physical contact when disconnecting lines/hoses after unloading. Disposal, Spill, or Leak Procedures In case of spills or leaks, wash with copious amounts of water. Ventilation is required in enclosed areas handling ammonia. Should a release occur in an enclosed area, eyes and skin should be protected from contact. All stationary storage installations shall have at least two suitable gas masks in readily accessible locations. Full face masks with ammonia canisters, for example, as approved by the Bureau of Mines, U.S. Department of the Interior, are suitable for emergency action for most leaks, particularly those that occur outdoors. For protection in concentrated ammonia atmospheres, self-contained breathing air apparatus is required. Stationary storage installations shall have an easily accessible shower or a 55gallon drum of water available. Fire Ammonia is considered to be nonflammable, but a large and intense energy source can cause ignition and/or explosion. Lower flammable limit for ammonia is 15.5% volume in air; the upper flammable limit is 27.0% volume in air; and the autoignition temperature is 1204⬚C (651⬚C). In case of fire, the best procedure is to stop the flow of gas. If the fire is small, use dry chemical or CO2; for large fires, use water spray, fog, or foam. Use water to keep fire-exposed containers cool and water fog or foam to reduce vapor concentrations if necessary. Full protective equipment, including a self-contained breathing apparatus, should be worn in a fire involving this material.5 References 1. Anon, “Ammonia and Ammonia Solution—Handling and Storage” (Sioux City, IA: Terra Industries Inc., 2002), 28 pp. 2. Anon, “Ammonia,” MSDS G-11 (Murray Hill, NJ: BOC Gases, 1996), http:// www.vngas.com/pdf/g11.pdf. 3. J.R. Jennings, ed., Catalytic Ammonia Synthesis (New York, NY: Plenum Press, 1991), pp. 393–394. 12 Materials Selector for Hazardous Chemicals 4. T.C. Collocott, A.B. Dobson, eds., Chamber Science and Technology Dictionary (Edinburgh, Scotland: W&R Chambers Ltd, 1984), p. 288. 5. Anon, “Aqua ammonia 19–30% NH3,” MSDS 2050 (Sioux City, IA: Terra Industries Inc., 2002), http://www.terraindustries.com/our_products/ammonia/ msds/aqua_ammonia_19–30.pdf. 6. Anon, “Aqua ammonia,” MSDS 11_01 (North York, ON, Canada: MARSULEX Inc., 2002), http://www.marsulex.com/PDF_MLX/MSDS/Ammonium Hydroxide.msds.pdf. 7. Anon, “Ammonia (Anhydrous) Chemical Backgrounder,” NSC, National Safety Council (2003), http://www.nsc.org/library/chemical/ammonia.htm. 8. R.J. Lewis, ed., Sax’s Dangerous Properties of Industrial Materials, 10th ed. (New York, NY: John Wiley & Sons, 2000), pp. 228–229. 3 Production of Ammonia Modern industrial synthesis of ammonia was first developed by Fritz Haber, a professor at Karlesrühe University, and Karl Bosch, an engineer with Badischer Anilinund Soda-Fabrik (BASF). Production of 30 metric tons per day began in 1913 near BASF’s Ludwigshafen complex. The Haber-Bosch process involved the reaction of gaseous nitrogen and hydrogen at high temperatures and pressures over iron catalysts. This development was important in that it replaced nitrogen sources such as Chilean nitrates and the limited production of ammonia from coke-oven gas. Local sources of nitrogen were needed at that time not only for agricultural uses but also in the manufacture of explosives.1 Ammonia is basically produced from water, air, and energy. The source of the energy is normally hydrocarbons, thus providing hydrogen as well as energy, but coal or electricity can also be used. Steam reforming of light hydrocarbons is the most efficient route, with about 77% of world ammonia capacity being based on natural gas. There are, however, many developments taking place largely to reduce energy use, increase production, and decrease plant size. Materials developments have greatly improved operation in the high-temperature parts of the process. Other innovations include gas-heated reforming and isobaric production, made possible by improvements in process design and catalysts.2,3 The typical size of a large single-train ammonia plant is 1,000–1,500 t/d, although capacities of 1,800 t/d and above are not uncommon for new plants. The process and energy systems are integrated to improve overall energy efficiency. The ammonia plant may stand alone or be integrated with other plants, such as a urea or nitric acid plant. A typical modern ammonia plant, showing the major items of equipment, is shown in Figure 3.1. Two main types of production process for ammonia synthesis gas are currently in operation in Europe: • Steam reforming of natural gas or other light hydrocarbons (natural gas liquids, liquefied petroleum gas, naphtha). Modified steam reforming using excess air in the secondary reformer and heat exchange autothermal reforming are also being used to some extent. This process is shown schematically in Figure 3.2.4 13 14 Materials Selector for Hazardous Chemicals Figure 3.1 General View of Ammonia Production Plant (photo courtesy of Sasol Ltd, Secunda, RSA) Figure 3.2 Block Diagram of the Steam/Air Reforming Process for Ammonia Production MS-6: Ammonia and Caustic Soda Figure 3.3 15 Block Diagram of the Partial Oxidation Process for Ammonia Production • Partial oxidation of heavy fuel oil or vacuum residue. This process is shown schematically in Figure 3.3.5 The ammonia synthesis process is largely independent of the type of synthesis gas production process, but synthesis gas quality influences the design and operating conditions. About 85% of world ammonia production is based on conventional steam reforming. The theoretical process conversions, based on methane feedstock, are given in the following approximate formulae: 0.88CH4 Ⳮ 1.26Air Ⳮ 1.24H2O r 0.88CO2 Ⳮ N2 Ⳮ 3H2 N2 Ⳮ 3H2 r 2NH3 (1) The synthesis gas production and purification normally take place at 25–35-bar pressure. The ammonia synthesis pressure is usually in the range from 100 to 250 bar. Since most of the catalysts used in the process are sensitive to them, sulfur and sulfur compounds are removed in the primary reformer section. Here the sulfur in the feed gas is hydrogenated to H2S. The desulfurized gas is mixed with process steam, and the steam/gas mixture is then heated to 500⬚C–600⬚C in the convection section before entering the primary reformer. The primary reformer consists of a large number of high–nickel chromium alloy tubes filled with nickel-containing reforming catalyst. The overall reaction is highly endothermic, and additional heat is required to raise the temperature to 780⬚C–830⬚C at the reformer outlet. 16 Materials Selector for Hazardous Chemicals Only 30% to 40% of the hydrocarbon feed is reformed in the primary reformer because of the chemical equilibria at the actual operating conditions. The temperature must be raised to increase the conversion. This is done in the secondary reformer by internal combustion of part of the gas with the process air, which also provides the nitrogen for the final synthesis gas. The process air is compressed to the reforming pressure and heated further in the primary reformer convection section to around 600⬚C (1112⬚F). The process gas is mixed with the air in a burner and then passed over a nickel-containing secondary reformer catalyst. The reformer outlet temperature is around 1000⬚C (1832⬚F), and up to 99% of the hydrocarbon feed (to the primary reformer) is converted, giving a residual methane content of 0.2% to 0.3% (dry gas base) in the process gas leaving the secondary reformer. The process gas is cooled to 350⬚C to 400⬚C (662⬚F–752⬚F) in a waste heat steam boiler or boiler/superheater downstream from the secondary reformer. The CO, CO2, and steam are removed from the gas leaving the secondary reformer. Remaining CO or CO2 is poisonous to the ammonia synthesis catalyst, so it is converted to methane by catalytic reaction with hydrogen. Water is also formed and is condensed/absorbed before the hydrogen/nitrogen syngas is compressed before ammonia synthesis that takes place on an iron catalyst at pressures usually in the range 100–250 bar and temperatures in the range 350⬚C to 550⬚C (662⬚F– 1022⬚F). Only 20–30% is reacted per pass in the converter because of the unfavorable equilibrium conditions. The ammonia that is formed is separated from the recycle gas by cooling/condensation, and the reacted gas is replaced with fresh makeup synthesis gas, thus maintaining the loop pressure. Since the reactions are exothermic and there is a large temperature range in the loop, extensive heat exchange is required. A newly developed ammonia synthesis catalyst containing ruthenium on a graphite support has a much higher activity per unit of volume and has the potential to increase conversion and lower operating pressures. Other catalysts based on cobalt and ruthenium are being investigated. The high-purity anhydrous ammonia from this stage is either used directly in downstream plants or transferred to storage tanks. From these, the ammonia can be transferred to road tankers, rail tank cars, or ships (see Chapter 8). The partial oxidation process is used in the gasification of heavy feedstocks such as residual oils and coal. Extremely viscous hydrocarbons and plastic wastes may also be used as fractions of the feed. An air separation unit is needed for the production of oxygen for the partial oxidation step. The nitrogen is added in the liquid nitrogen wash to remove impurities from the synthesis gas and to get the required hydrogen/nitrogen ratio in the synthesis gas. The partial oxidation gasification is a noncatalytic process taking place at high pressure (⬎50 bar) and temperatures around 1400⬚C (2552⬚F). Some steam is added for temperature moderation. The gas is treated to remove minerals as ash, sulfur as hydrogen sulfide, soot, and carbon dioxide. The ammonia synthesis is similar to that used in steam reforming plants but simpler and more efficient because of the high purity of synthesis gas from liquid nitrogen wash units and the synthesis loop not requiring a purge.6 Showa Denko of Japan has constructed a 65,000 t/a ammonia unit in the indus- MS-6: Ammonia and Caustic Soda 17 trial city of Kawasaki near Tokyo based on gasification of municipal plastic wastes. As well as ammonia production, the plant will recycle sulfur, metallic impurities will be recovered and sold, and chlorine will be captured.7 This plant started production in 2003.8 References 1. Anon, “Ammonia Synthesis,” University of Idaho (2003), http://www.chem. uidaho.edu/⬃honors/ammonia.html. 2. P. Agarwal, “Ammonia: The Next Step,” Chemical Engineers Resource Page (2002), http://www.cheresources.com/ammonia.shtml. 3. Anon, “KAAP plus威—Advanced Technology for Low-Cost Ammonia Production,” brochure no. HO2369 (Houston, TX: Kellogg Brown & Root, 2000), 2 pp. 4. Anon, “Production of Ammonia,” vol. 1 (Brussels, Belgium: EFMA, European Fertilizer Manufacturers’ Association, 2000), p. 10. 5. Anon, “Production of Ammonia,” vol. 1 (Brussels, Belgium: EFMA, European Fertilizer Manufacturers’ Association, 2000), p. 11. 6. Anon, “Production of Ammonia,” vol. 1 (Brussels, Belgium: EFMA, European Fertilizer Manufacturers’ Association, 2000), 44 pp. 7. Anon, India Fertilizer News (Aug. 2003), http://www.fertindia.com/ International%20News-.htm. 8. Anon, “Message from the Management—Annual Report 2002” (Japan: Showa Denko, Aug. 2003), www.sdk.co.jp/contents/investment_info. 4 Corrosion by Ammonia Corrosion is the deterioration of a material by reaction with its environment. For metals and alloys, this is mostly an electrochemical process involving anodic and cathodic reactions. Corrosion rates in aggressive chemicals usually decrease as the pH increases. In alkaline solutions, the hydrogen ion is present in very low concentrations. However, many metals pass through a minimum corrosion rate at some pH, usually basic, and then suffer increased corrosion as pH continues to rise. Aluminum can liberate hydrogen ions from basic solutions. Since hydrogen ions are in short supply, it is likely that the cathodic reaction in alkaline media involves absorbed water molecules, such as H2O Ⳮ e r OHⳮ Ⳮ H (1) while the anodic reaction remains the same: M r MⳭ Ⳮ e (2) The metal ion is removed from solution by forming a basic salt such as ferrate, aluminate, or zincate. In ammonium hydroxide, nickel and copper will dissolve to form complex amines. Quite often, corrosion by alkalis leads to pitting and other localized attack because they tend to form cathodic films, and attack is concentrated at susceptible anodic areas. Passivity Stainless steels and some other iron-chromium and nickel-chromium alloys, as well as aluminum, titanium, and zirconium, develop a relatively inert, tenacious surface oxide layer. Interruptions to this film by inclusions, embedded metal particles, or mechanical breaks provide unprotected sites for corrosion to occur. 19 20 Materials Selector for Hazardous Chemicals In the case of austenitic stainless steels, inclusions and other foreign materials can be removed from the surface by immersion in 10% nitric acid with 3% hydrofluoric acid at about 70⬚C (158⬚F). The purpose of this treatment is to clean the metal surface and remove embedded particles before allowing the oxide film to reform in a more continuous, thicker, and more tenacious form than might occur naturally. A similar process involving the use of caustic solutions is applied to aluminum alloys. The protective surface oxide reforms spontaneously on reexposure to air. The terms “active” and “passive” are used to describe the corrosion behavior of metals that form these protective films. When active, the protective oxide film is breached or removed, and corrosion proceeds on the base material. When passive, the protective oxide film remains intact and minimizes corrosive activity. Forms of Corrosion Several types of corrosion are encountered in ammonia production plants, including uniform corrosion, galvanic corrosion, acid attack, crevice corrosion, intergranular corrosion, cavitation corrosion, high-temperature corrosion, and corrosion related to hydrogen, embrittlement, metal dusting, and so on. These corrosion problems happen at various stages in the production process.1 General Corrosion General or uniform corrosion is the common form of metal loss in most corrodents in the absence of passivating films. In this form of corrosion, the metal is removed uniformly over the entire exposed surface, and a corrosion allowance can be built into the design based on expected corrosion rate. Pitting Corrosion Pitting is a localized type of corrosion usually taking the form of small but deep cavities. Pits tend to originate at points where the protective oxide film is breached. Mechanical damage and surface deposits are two reasons for the development of active areas leading to pits. Once started, the pit develops its own corrosive environment and proceeds independently of the bulk solution. Chlorides are often involved in pitting corrosion of stainless steels. Pitting in aluminum initiates at flaws in the oxide film, usually associated with intermetallic phase particles in the aluminum. Chlorides are often but not always associated with pitting corrosion in aluminum.2 MS-6: Ammonia and Caustic Soda 21 Crevice Corrosion Crevice corrosion is induced by a similar mechanism to pitting, that is, breakdown of the passive film leading to active localized corrosion. Crevice corrosion occurs on closely abutting surfaces, such as flange faces and under gaskets where the bulk liquid cannot penetrate. Chemical changes within the crevice due to depletion or accretion of aggressive species cause pitting and attack within the crevice. The area outside the crevice comprises the cathode, while that internal to the crevice comprises the anode in the circuit. Crevice corrosion is often associated with oxygen depletion within the crevice. Intergranular Attack Intergranular attack (IGA) is a form of accelerated corrosion in grain boundary regions caused by compositional differences. IGA occurs in austenitic stainless steels because of a depletion of chromium in this region. These chemical differences within the alloy result from thermal processing in a temperature range, usually 425⬚C to 815⬚C (800⬚F–1500⬚F) favoring chromium carbide precipitation at the grain boundaries. The stainless steel is then said to be “sensitized” (i.e. susceptible to IGA). Erosion-Corrosion This is a form of corrosion resulting from the loss of protective surface oxide from excessive velocity or turbulence of contacting fluids. It can also be caused or accelerated by the presence of entrained particles. Centrifugal pumps often exhibit this type of corrosion. This problem is controlled by alloy selection. Stress Corrosion Cracking Stress corrosion cracking (SCC) is an environmentally assisted form of attack that results from interaction between the environment and a specific alloy system under tensile stress. It is a temperature-sensitive phenomenon. Classical examples are SCC of copper alloys in ammonia, steel in caustic soda, and stainless steels in chloride environments. Carbon and low-alloy steels can suffer from SCC in ammonia, and steps must be taken to avoid or control this problem (see Chapter 5 for details). Vapor-Phase Attack Most corrosion data are derived from laboratory tests made in the liquid phase. However, in actual plant equipment, there is often a vapor space above the liquid 22 Materials Selector for Hazardous Chemicals acid where vapor composition may differ from the bulk liquid. This vapor may condense on the equipment inner surface and be either more or less corrosive than the bulk liquid. The condensing liquid may not contain constituents that inhibit attack in the bulk liquid phase. This occurs in anhydrous ammonia storage in which small quantities of water prevent SCC in the liquid but may be absent in the vapor (see Chapter 5 for details). High-Temperature Corrosion Since many parts of ammonia production plant operate at elevated temperatures, they are prone to a number of high-temperature corrosion and embrittlement mechanisms. These include oxidation, nitriding, hydrogen attack, carburization, metal dusting, hydrogen sulfide corrosion, and temper embrittlement. These forms of corrosion are normally controlled by a combination of selected operating conditions and materials that are used in the parts of the plant where these forms of deterioration can occur (see Chapter 5 for details). Dealloying Dealloying is the term used to describe the preferential loss of one component of a multicomponent alloy. In anhydrous ammonia, the phenomenon is not common, but some copper-based alloys are susceptible to dealloying in ammonia solutions. References 1. M.P. Sukumaran Nair, “Tackling Corrosion in Ammonia Plants—Selecting the Proper Materials,” Chemical Processing 12, 1 (2001). 2. E.E. Stansbury, R.A. Buchanan, Fundamentals of Electrochemical Corrosion (Materials Park, OH: ASM International, 2000), p. 325. 5 Corrosion of Metals and Alloys This chapter discusses the corrosion behavior of metals and alloys in nominally pure ammonia and ammonium hydroxide. Corrosion in contaminated solutions and mixtures is discussed in Chapter 7. Materials of construction for specific plant items and types of equipment are described in Chapter 8. Ammonia and ammonium hydroxide are not particularly corrosive in themselves, but some problems arise with specific materials, particularly in the presence of contaminants. Air or oxygen contamination is a factor in many instances, causing general corrosion of some materials and localized corrosion, specifically stress corrosion cracking, in others. Contamination with carbon dioxide can lead to corrosion due to carbamates, sometimes encountered in ammonia recovery systems. Hightemperature corrosion will occur in hot dissociated ammonia. Aluminum and Its Alloys Aluminum and its copper-free alloys show good resistance to dry, gaseous ammonia at ambient or elevated temperatures. Corrosion rates of ⬍0.025 mm/y (1 mpy) at 21⬚C and ⬍0.05 mm/y (2 mpy) at 100⬚C are typical.1 Aluminum may be used for cargo tanks for the anhydrous product. If moisture is present, there is some attack on aluminum, but a protective film soon forms and corrosion stops. Aluminum tubing is used in ammonia refrigeration operating in liquid ammonia containing 5% water. In moist ammonia, vapor corrosion is low, below about 50⬚C (120⬚F). Under condensing conditions of steam and ammonia, aluminum can be attacked, and the rate does not decrease with time. Attack is prevented if the CO2:NH3 ratio is at least 2.5:1. Hydrogen sulfide also inhibits corrosion under condensing conditions. Aluminum is used for compressors, heat exchangers, evaporators, condensers, and piping in the production of ammonia. Aluminum pressure vessels are used in the storage and transport of ammonia.2 There is mild action on aluminum in ammonium hydroxide solutions at temperatures below about 50⬚C (120⬚F). The greatest attack occurs in concentrated so23 24 Materials Selector for Hazardous Chemicals lutions (around 25%) and at about 5% concentration. For aluminum to perform well in ammonium hydroxide, the solution must be free from heavy metals and halogen ions. Attack is limited if the solution is saturated with aluminum ions before exposure takes place. As with anhydrous ammonia, aluminum is attacked at higher temperature, but this attack stops as a protective film forms.3,4 Even at ambient temperatures, corrosion rates decrease with time of exposure (see Table 5.1).5 The initial attack of aluminum by dilute ammonia solutions (up to ⬃10%) is controlled by the diffusion of OH ions to the surface and is a function of pH. The surface is passivated once sufficient corrosion product has been produced to form a protective film. Under some exposure conditions, the corrosion product may continue to dissolve rather than form a protective film.2 Aluminum alloys are highly susceptible to the effects of contaminants and can suffer pitting attack. Any application for aluminum should include testing of samples under the exact service conditions expected. Table 5.1 Corrosion Rates of Aluminum in Ammonium Hydroxide Solutions at 20⬚C (68⬚F) Concentration 2% Corrosion rates in g/m2/d (mm/y) 1-day test 130 (16.9) 7-day test 29 (3.8) 5% 10% 22% 176 (22.9) 35 (4.6) 159 (20.7) 33 (4.3) 63 (8.2) 10 (1.3) Iron and Steel Ferrous alloys are generally not corroded by ammonia or ammonium hydroxide at ambient or elevated temperatures. Corrosion can, however, occur in the presence of contaminants, and they can be subject to stress corrosion cracking in storage at ambient temperatures and can become embrittled in low-temperature handling. Cast Irons Cast iron has been widely used in ammonia production and handling. Since it is generally thick walled, somewhat higher corrosion rates can be tolerated as long as the iron in solution is acceptable. Cast iron strippers have been used to concentrate crude gas liquors with about 1–2% ammonia up to 25% ammonium hydroxide. A corrosion rate of 1 mm/y (40 mpy) has been found in the vapor space of a nodular cast iron ammonia pressure distillation plant operating at about 88⬚C (190⬚F). White cast iron with ⬎18% chromium content, ASTM A532 Grade IID, has been used in some applications. MS-6: Ammonia and Caustic Soda 25 High-nickel cast irons have been used in valves, pumps, and so on in ammonium hydroxide solutions. The austenitic nickel cast iron NiResist威 cooling coils corroded at about 0.005 mm/y (0.1 mpy) at 25⬚C (77⬚F) and 0.15 mm/y (5.9 mpy) at 70⬚C (158⬚F) in flowing ammonia gas.1 Laboratory testing of NiResist威 cast irons and unalloyed cast irons gave the results shown in Table 5.2.6 This table also shows data from ammonia-containing environments in industrial applications. In most applications, unalloyed cast iron is at least as good as the NiResist威 irons, but in the concentrated and contaminated solutions, the alloyed irons are superior. In most cases in this table, the recommended NiResist威 are types 1 (F41000) and 2 (F41002) with type 3 (F41004), also appropriate for the dilute solutions. Table 5.2 Corrosion Data for NiResist威 and Cast Iron in Ammonia Solutions and Environments Medium 5% ammonium hydroxide 10% ammonium hydroxide 25% ammonium hydroxide 50% ammonium hydroxide 75% ammonium hydroxide Concentrated ammonium hydroxide Ammonia 5%–6% by vol; 150 ppm phenol in H2O vapor Ammonia liquor separator tank Ammonia liquor 6.5 g/L ammonia Ammonia liquor with sulfates, sulfides, etc. Average Corrosion Rate (mpy [mm/y]) Temperature (⬚F [⬚C]) Cast Iron NiResist威 60 (15.6) 60 (15.6) 60 (15.6) 60 (15.6) 60 (15.6) 60 (15.6) 215.6 (102) Nil Nil Nil Nil Nil 2 (0.05) 2 (0.05) 0.01 0.2 (⬍0.01) 0.18 (⬍0.01) Nil Nil Nil 0.9 (0.02) — 215.6 (102) 100 (37.8) 0.09 (⬍0.01) 3 (0.08) 0.1 0.05 (⬍0.01) 0.6 0.01 Carbon Steels Ammonia is essentially noncorrosive to steels at ambient temperatures, which accounts for their widespread use. Carbon steels (commonly referred to as “steels” in many standards and regulations pertaining to ammonia storage and transport) include both carbon- and carbon-manganese steels commonly specified for process equipment. These alloys are by far the most commonly used materials for the storage and handling of ammonia. The standard used to specify the steel is selected on the basis of the mechanical properties required by the application, with consideration of environmental factors. Even at elevated temperatures, corrosion rates in anhydrous ammonia are low, less than 0.05 mm/y (1.9 mpy) in the range from 297 to 589 K (24⬚C–316⬚C).7 One 26 Materials Selector for Hazardous Chemicals very important exception relative to corrosion resistance of steels is the tendency of anhydrous ammonia to cause stress corrosion cracking (see discussion later in this chapter). Ordinary carbon and alloy steels are satisfactory in ammonium hydroxide service, although a superficial rusting will occur in the vapor space. Stress Corrosion Cracking of Steels Ammonia stress corrosion cracking (SCC) in carbon-steel vessels was first reported in the mid-1950s in agricultural service tanks. Cracking occurred in areas of high residual stress, such as welds and cold-formed dished heads. Hot forming or stress relieving the heads considerably reduced the occurrence of cracking, as did the addition of a small amount of water to the ammonia. Throughout the 1960s and early 1970s, cracking problems appeared to be associated mainly with high-strength quenched and tempered steels. Later there were reports of cracking occurring in spheres containing anhydrous ammonia with water additions and also in spheres that had been stress relieved after finding and repairing cracks. The cause of this cracking is now accepted to be high local stresses and the presence of air contamination, although nitrogen and carbon dioxide are also thought to play a role. Cracking is accelerated by the use of high-strength steels, the presence of hard welds, and air contamination.2,8 The highest susceptibility to SCC has been found to be in liquid ammonia with 3 to 10 ppm oxygen and a water content ⬍100 ppm. SCC can occur, however, in ammonia with an oxygen content down to 0.5 ppm when the water content is very low.9 Possible ways to control or reduce liquid ammonia stress corrosion cracking in carbon steels include the following:10 • • • • • • • • • Eliminate oxygen Add around 0.2% water to the ammonia Use steel with an actual yield strength less than 300 MPa Reduce residual stress by stress relieving Inspect often enough to detect cracks before they grow to dangerous proportions Use a sacrificial anode such as a zinc spray Cathodically protect the steel Use a different material such as stainless steel Paint the steel with a suitable protective coating Some of these options can be eliminated for the following reasons: • There is no commercially available paint suitable for ammonia submersion duty. • Using stainless steel would increase the capital cost of vessel construction by at least 100% and increase steel mass. • Cathodic protection has not yet been shown to be reliable in refrigerated installations and would not protect in vapor spaces (e.g. in road tankers). • Zinc spraying of ammonia tank internals has been used, largely on a trial basis. More experience is required. MS-6: Ammonia and Caustic Soda 27 The remaining options that are currently used for storage vessels or road tankers are evaluated in Table 5.3.10 Despite all the research and the available information on the subject of SCC of steels in ammonia, sudden failures do still occur. A 20-year-old liquefied ammonia gas cylinder with a charged capacity of 25 kg operating at 12-bar pressure fractured into four pieces. These cylinders are hydraulically tested annually at 30 kg/cm2. The failure was found to have initiated in the upper dished head, near to the girth weld. A similar unfailed cylinder was destructively examined and found to have SCC cracks in the same location. It was recommended that all cylinders with similar operating history should be scrapped, that this type of cylinder should not be exposed to direct sunlight, and that they should be regularly inspected to applicable standards.11 SCC of carbon steel occurred in ammonia receiver tanks used for recycling ammonia in a urea plant. These tanks (SA 516 grade 70) suffered extensive cracking in welds and HAZ in spite of the addition of 0.2% water to the system. It was concluded that the water was not uniformly distributed throughout these vessels and was, anyway, probably inadequate to protect the vapor space and in the condensing ammonia.12 Nitriding of Steels At high temperatures, ammonia may dissociate into hydrogen and nascent nitrogen. The nascent nitrogen has a high affinity for iron and reacts to form a very hard, brittle metal nitride. This is sometimes a desirable reaction, and wear-resistant surfaces are commonly produced on steel parts by nitriding in an ammonia atmosphere. Although commercial nitriding is performed at temperatures above the normal service temperature for steels (495⬚C–565⬚C or 925⬚F–1,050⬚F), significant nitriding can occur at lower temperatures, resulting in loss of ductility. Ammonia dissociation is catalyzed by iron, which also contributes to the damage potential. For these reasons, steels are restricted to use at temperatures below 300⬚C (600⬚F) in ammonia service. In ammonia converters, nitriding layers can develop over time to a depth of several millimeters, and these hard layers can cause brittle, surface cracks to form. Austenitic steels (e.g. in converter baskets) develop thin, hard nitrided layers that tend to flake off.13 Hydrogen Attack At high temperatures and pressures, hydrogen that is present in ammonia synthesis can dissociate, the atomic hydrogen entering the steel lattice to react with carbon to form methane. This weakens the steel in two ways: by removing carbon from the steel (decarburization) and by forming blisters or fissures in so doing. This can eventually cause the vessel or pipe to rupture, often without any obvious prior deformation. Areas around weld seams are particularly prone to this phenomenon. The risk of attack may exist at temperatures as low as 200⬚C (392⬚F) and hydrogen partial pressure as low as 7 bar. Selection of appropriately resistant materials can largely eliminate this problem. The classic Nelson curves give guidance on the stability limits of various alloys in terms of temperature and hydrogen partial pressure. These original guidelines have been modified in recent years on the basis of ongoing 28 Materials Selector for Hazardous Chemicals Table 5.3 Ammonia SCC Mitigation Measures Corrosion Mitigation Scheme Eliminate oxygen Passivate ammonia with 0.2% water Use steel with ⬍300 MPa actual yield strength Stress relieve Inspect regularly Refrigerated Tanks This is a normal commissioning procedure, but regular inspection reintroduces oxygen. The number of inspections should be minimized; online inspection is desirable. This is now normal practice. Most tanks are constructed of grade 490 steel (ASTM A515 or equivalent), so actual yield easily exceeds 300 MPa. Not practical. Traditionally tanks were shut down to inspect. Inspection online now becoming more possible and used. Road Tankers Not usually practical. Vapor space not protected. Tankers in Australia are often made of quenched and tempered steel where yield ⬎600 MPa to reduce tare weight. Mandatory, especially where yield ⬎300 MPa, which is almost always. Inspection is mandatory, usually every 1 or 2 years. experience. For example, low-alloy steels with 0.25% and 0.5% molybdenum are now classed as unalloyed steels from the point of view of resistance to hydrogen attack.13 Steels with low levels of molybdenum and no chromium failed in a number of cases in catalytic reforming service, so care was recommended in the use of these alloys for that application. However, failures caused by hydrogen also occurred in hydrodesulferization, ammonia synthesis, and other parts of ammonia production plants, leading to their removal from these safe operating charts.14 Curves that incorporate findings from the extensive operating experience are available that provide safe operating conditions for carbon and low-alloy steels (see Figure 5.1).15,16 The standard chromium-molybdenum steels are being modified and improved so that they can be used at higher temperatures. A modified 21/4% Cr, 1% Mo has been developed that is usable at temperatures up 484⬚C (903⬚F) instead of being limited to 454⬚C (849⬚F) as are conventional steels of this type, and this higher temperature operation is permitted under ASME II rules. The modification included the addition of 1/4% V. The API 941 standard has placed this modified steel at the same limits in terms of temperature and hydrogen as the 3% Cr, 1% Mo steel. The first MS-6: Ammonia and Caustic Soda 29 Figure 5.1 Operating Limits for Steels in Hydrogen Service, API 941 vessel made from this steel was an ammonia converter fabricated in 1995. The vanadium modified steel also has a better resistance to temper embrittlement than does the standard, unmodified version.16 Hydrogen damage can also occur from the dissociation of molecular hydrogen into atomic hydrogen at elevated temperatures. Atomic hydrogen can diffuse into metal structures and recombine into molecular hydrogen at defects or discontinuities within the metal. This is also known as hydrogen embrittlement and is usually associated with welds that have not received correct PWHT. In contrast to the phenomenon described previously, this effect of hydrogen is reversible, and hydrogen can diffuse back out of the structure if held at atmospheric pressure at around 300⬚C (572⬚F). Slow cooling from elevated temperature and pressure operation is often recommended to permit this diffusion process to occur. Temper Embrittlement If heat-resistant steels are held at tempering temperatures, that is, above about 400⬚C (752⬚F), for long periods, their impact properties can decline. The transition temperature between ductile and brittle behavior can be elevated to 60⬚C (140⬚F) from the normal zero or below. This tendency to embrittle can be reduced by controlling the level of trace elements (silicon, phosphorus, manganese, and tin) in the steel. In this respect, modern steels are generally much cleaner than some of the older steels and so are less prone to this phenomenon. Vessels or pipes in which temper embrittlement is anticipated should not be pressurized at low temperatures. Hydrogen Sulfide Attack Most high-temperature steels are attacked by hydrogen sulfide in the gas stream in partial oxidation plants. The use of austenitic stainless 30 Materials Selector for Hazardous Chemicals steels eliminates this problem, but stress relief of welds is advised in these plants to avoid SCC by chlorides sometimes present in the feed oil.13 Alloy Steels Steels alloyed with small amounts of chromium, molybdenum, nickel, or other elements are referred to as low-alloy steels, and they can provide an economical alternative to high-alloy steels at both high and low temperatures. Low-alloy steels have been used effectively in ammonia storage vessels. These steels are also subject to SCC, with high stresses and air contamination being the main factors causing cracking. The addition of 0.1% to 0.2% water inhibits this attack.2 Chromium molybdenum steels are the most common steels of this type used in ammonia applications. Chromium-Molybdenum Steels Alloy chromium-molybdenum steels are commonly used at elevated temperatures where the alloying additions provide resistance to hydrogen attack and increase the strength of the alloy. Typical alloys are 1.25% Cr, 0.5% Mo (K11597); 2.25% Cr, 1% Mo (K21950); 5% Cr, 0.5% Mo (K41545); 7% Cr, 0.5% Mo (S50300); and 9% Cr, 0.5% Mo (S50400). Their use in ammonia service is chiefly in ammonia synthesis, the alloy selection being based on API Publication 941.15 Old editions of this standard have curves showing the resistance of carbon, 1/2% molybdenum alloys (with no chromium addition). As mentioned previously under hydrogen resistance, service experience has shown that this alloy has little more resistance to hydrogen attack than carbon steel, so the API publication was updated in 1991 to reflect this experience. Other uses for these chromiummolybdenum steels are in high-strength parts such as fasteners. Nickel Alloy Steels Addition of nickel to steel greatly enhances the lowtemperature toughness (impact properties). For this reason, these materials are sometimes specified for low-temperature ammonia service, especially as weld filler metals. Typically, the nickel alloy steels contain 3.5% (K32025), 6%, or 9% nickel (K81340). Stainless Steels Stainless steels are not normally required in ammonia, from the point of view of corrosion, but they do find many applications. All grades of stainless steel are resistant to ammonium hydroxide solutions at up to the atmospheric boiling point. Stainless steels can be classified into three groups according to metallurgical structure and response to heat treatment. These are the martensitic, ferritic, and austenitic groups. Further subdivisions include duplex alloys with austenitic/ferritic microstructures and precipitation-hardening (PH) grades strengthened by an agehardening treatment. MS-6: Ammonia and Caustic Soda 31 Ferritic Grades Ferritic stainless steels show a transition from ductile to brittle behavior over a narrow temperature band. This transition can occur above room temperature in steels with high levels of carbon, nitrogen, or chromium. This effect, combined with a tendency to sensitize from heating, limited the usefulness of the early ferritic stainless steels and restricted them to thin sections. The modern low-carbon, low-nitrogen grades (with or without stabilizing additions) have limited toughness and are still usually restricted to thin sections. The ferritic stainless steels are resistant and sometimes immune to chloride stress corrosion cracking. This type of alloy can be subject to 475⬚C (857⬚F) embrittlement caused by precipitation of ␣⬘ chromium–rich phase. The ferritic grades are also particularly prone to r-phase precipitation because of their high chromium and molybdenum contents. Both types of embrittlement can be removed by heating and rapid cooling. The first superferritic steels were based on 26% Cr, 1% Mo (S44627) and the columbium stabilized XM-27 (S44627). These were developed to provide better resistance to chloride SCC than the austenitic 300 series. These ferritic steels have low interstitial content with high chromium and very low carbon levels. Most modern superferritic stainless steels are based on a 29% Cr, 4% Mo alloy, and they need low C Ⳮ N levels (i.e. less than 0.025%) to avoid intergranular corrosion caused by chromium depletion from precipitation of carbides and nitrides. Some of the current ferritics contain higher levels of C Ⳮ N and have additions of titanium or niobium as carbon/nitrogen stabilizers. Superferritic steels include AL 29-4C威 (S44735), AL 29-4-2威 (S44800), Sea-Cure威 (S44660), and Monit威 (S44635). The superferritic AL 29-4C威 (S44735) has been used successfully in an ammonia stripper reboiler handling water, ammonia, H2S, and steam.17 Precipitation-Hardening Grades The PH stainless steels see limited service in ammonia environments but play an important role nonetheless. These alloys exhibit very high strengths combined with good notch toughness and corrosion resistance properties. For this reason, they are often the preferred material for such parts as valve stems and certain critical fasteners. Some of these alloys are susceptible to hydrogen embrittlement in corrosive or hydrogen-rich environments. Duplex Stainless Steels Duplex stainless steels have a controlled balance between austenite- and ferritebearing constituents. The “duplex” structure contains approximately 50/50 austenite and ferrite phases, resulting in higher strength as compared to the 18-8 grades, 32 Materials Selector for Hazardous Chemicals as well as improved corrosion resistance in some environments (e.g. chloridebearing aqueous solutions). Duplex stainless steels are not commonly used in ammonia service except where their resistance to chloride SCC is useful from the water side of heat exchangers. The modern grades, such as alloy 2205 (S31803), typically also contain molybdenum to achieve a composition of about 22% Cr, 5.5% Ni, 3% Mo, 0.03% C max. They are strengthened and stabilized by nitrogen. The mixed austenite-ferrite structure imparts strength and resistance (but not immunity) to chloride pitting and SCC. The duplex 3RE60 (S31500) has been used to replace standard austenitic stainless steels in this type of situation. The duplex stainless steels have higher strength than the lower austenitic grades such as type 304 (S30400) but are subject to temper embrittlement at about 475⬚C (885⬚F). Welding tends to lead to variations (20%–80%) in the austenite/ferrite ration in the as-cast weld bead and fusion zone. To minimize this effect, they are welded with a nickel-rich, overmatching rod. Austenitic Stainless Steels The austenitic stainless steels constitute a large, diverse body of alloys developed from the original 18% Cr, 8% Ni stainless steel, type 302 (S30200). The standard commercial grade is type 304 (S30400), approximately 18% Cr, 8% Ni, ⬍0.08% C. Additions of titanium or columbium protect against sensitization and chromium carbide precipitation by precipitating carbon as titanium or columbium carbides, thus preventing chromium depletion. Types 321 (S32100) and 347 (S34700) are the common stabilized grades. A low-carbon type 304L (⬍0.03% C max.) version is available and is widely used to overcome the problems associated with chromium carbide precipitation and chromium depletion. A molybdenum-containing variant is type 316L (S31603), containing about 17% Cr, 12% Ni, and 2% to 3 % Mo. The molybdenum addition improves corrosion resistance in many environments, such as in chloride-containing solutions. There is also a stabilized version of the molybdenum-containing grade that is commonly used in Europe and is becoming more common in North America. This grade, 316Ti (S31635), is a high-carbon stainless steel with the carbon stabilized by the addition of a titanium addition equal to at least five times the carbon content. Its main application is in situations where it is exposed to temperatures between 550⬚C and 800⬚C (1022⬚F and 1471⬚F) for prolonged periods. Modern steelmaking practice has made it possible to produce L grade stainless steels with controlled nitrogen additions that have the mechanical properties of the equivalent high-carbon, non–L grade. Dual grade stainless steels 304/304L and 316/ 316L are now available and permitted for use in many applications. ASME has ruled that a dual grade steel may use the straight non–L grade allowable stresses for all forms up to 540⬚C (1004⬚F). Improved steelmaking control also means that 316 can be made more cheaply with the molybdenum content at the low end of the permitted range of 2% to 3%. This can have a serious effect on the corrosion resistance of this type of steel. In applications where molybdenum is a key factor in corrosion resis- MS-6: Ammonia and Caustic Soda 33 tance, a minimum level should be specified, or type 317 or 317L, with 3% to 4% Mo, should be used.18 The standard austenitic grades, such as types 304 (S30400) and 316 (S31600), find widespread use in ammonia service since they are resistant to general corrosion and ammonia stress corrosion cracking. Stabilized and low-carbon grades are not generally needed in ammonia applications. Type 304 (S30400) stainless steel was found to be resistant to intergranular attack in 28% ammonia solution at room temperature even after sensitizing for 1 hour at 677⬚C (1251⬚F).19 Stainless steels can, however, fail by SCC that is initiated by sensitization formed during heating, for example, when welding. Such a failure in a reformer tube occurred at the welded junction between the type 321 flange and HK 40 catalyst tube. The weld and HAZ was sensitized when joined using a high-carbon welding rod. The high–nickel alloy weld intended to protect the cast steel (HK 40) from corrosion had inadvertently been machined off, exacerbating the corrosion.20 The high cost of stainless steels relative to carbon and low-alloy steels often precludes their use for large equipment, but their many advantages lead to their selection for smaller parts and components. They are used in low temperature service since they have an extremely low Nil Ductility Transition Temperature (NDTT) and exhibit excellent notch toughness at temperatures far below the atmospheric boiling temperature of ammonia. In elevated temperature services in ammonia synthesis, they are used because of their resistance to hydrogen attack and nitriding. Some shallow nitriding does occur after years of service in aggressive environments, but because of the excellent notch toughness of the base material, this effect does not reduce the structural integrity. Another common area of application of the basic grades is in heat exchanger tubes, where the material selection is influenced by the fluid used to heat or cool the ammonia. These standard grades can suffer from chloride SCC, particularly in partial oxidation plants in which the feed oil contains chlorides. Chloride SCC failures can also occur under upset condition. An example of such a failure occurred in type 321 (S32100) and 310S (S31008) tubes in a waste heat boiler in an ammonium synthesis converter. The direct cause of this SCC was the failure of a feedwater boiler pump that was not repaired or replaced and permitted a buildup of deposits in the system. General and intergranular corrosion was also observed on these tubes.21 There are many more specialty grades of stainless steels that see service where some factor limits the use of other materials. A very common example is the use of special ferritic grades or duplex grades of stainless steels as heat exchanger tubes where austenitic stainless steels are subject to pitting or chloride stress corrosion cracking from cooling waters. The martensitic grades of stainless steel are used where high strength is required, especially at elevated temperatures, as in compressor rotors. Cast Stainless Steels The equivalent cast version of types 304 (S30400) and 304L (S30403) are CF8 (J92600) and CF3 (J92700), respectively, and they exhibit approximately the same corrosion 34 Materials Selector for Hazardous Chemicals response as the wrought alloys. However, castings can have surface layers containing more than the maximum allowable carbon content of 0.08%, which can significantly reduce corrosion resistance of the surface. In the cast form, the difference between CF8 (J92600) or CF3 and the corresponding molybdenum-bearing grades CF8M (J92900) and CF3M (J92800) is insignificant. CF 3 or CF 8 is not particularly common, and manufacturers of cast pumps and valves tend to standardize on CF 8M, which has a broader range of applications. The molybdenum-grade castings are often more available and cheaper than the 304or 304L-grade equivalents. Since the cast version of these alloys is unlikely to be welded, there is rarely a justification for specifying the low-carbon grades in this case. This assumes that the valves or pumps, if weld repaired by the manufacturer, are properly reheat treated (solution annealed) to restore optimum corrosion resistance. Availability and price are likely to favor the non–L grade, and a properly heat-treated casting in CF 8 or CF 8M is likely to be as corrosion resistant as their low-carbon cast or wrought equivalents. Alloys for Use at Elevated Temperatures Many of the operations in ammonia production take place at elevated temperatures. Some of the materials used for these applications are conventional iron-based or nickel-based alloys that have been discussed previously. However, these specialized elevated-temperature duties in ammonia production, petroleum refining, and so on have generated a demand for ever better materials specific to these duties. Many of the materials used here are proprietary and have been developed to have high strength and good creep resistant properties in aggressive, gaseous environments. The standard stainless steels, types 304, 310, and 347 (S30400, S31000, and S34700), were commonly used but often failed by cracking at elevated temperatures. A better alloy, HK40 (J94204) with 25% Cr, 20% Ni, was developed, and this became the industry standard.22 A later material development produced the HP (N08705) alloy with 26% Cr, 35% Ni, which was modified by the addition of alloy elements such as molybdenum, niobium, or tungsten. These modified alloys have improved creep resistance but still possess good ductility and weldability. Various HP-modified alloys are used in ammonia applications, one of the most common being HP 45Nb.5 A more recent development is the production of HP microalloys in which trace quantities of titanium, zirconium, and rare earths are added during casting. These alloys have better resistance to carburization and better high-temperature creep-rupture properties.22 Other new alloys, many of them proprietary, exist, such as 25% Cr, 35% Ni, 15% Co, 5% W; 28% Cr, 48% Ni, 5% W; and 35% Cr, 45% Ni plus additional elements, and these offer higher strength and good high-temperature properties.23 There has also been some development of coatings to resist high-temperature attack by ammonia. Uncoated types 304 (S30400) and 316 (S31600) and the same steels coated with silica applied by a sol-gel procedure were tested in anhydrous MS-6: Ammonia and Caustic Soda 35 ammonia at high temperature. The uncoated samples were attacked and formed a nitride scale that embrittled the metal. After 115 hours of testing at 500⬚C (932⬚F), uncoated samples were completely degraded, while the coated samples were only lightly attacked. Multilayer coatings were most effective, and the stainless steel substrates were not sensitized to intergranular attack by the high-temperature coating process.24 Creep Resistance Apart from metal loss due to corrosion or oxidation processes, the other important factor governing use of alloys is their mechanical properties. Creep is the process whereby strain or deformation occurs (usually at elevated temperature) under the application of stress levels below yield. A component under creep loading may eventually fail by a process known as stress rupture if the creep stress is not relieved by strain. Alloys discussed previously that have adequate resistance to this mechanical phenomenon and to high-temperature corrosive attack have been developed and are used in ammonia furnaces and other similar applications. Metal Dusting The phenomenon of metal dusting occurs during high-temperature operation, such as in steam superheaters downstream of the secondary reformer. It is related to the process of carburization, in which carbon migrates into the structure, forming hard carbides. Carburization occurs above about 800⬚C (1471⬚F) in the presence of hydrocarbons that crack to provide the carbon. Metal dusting occurs at 500⬚C to 800⬚C (932⬚F–1471⬚F) on iron-nickel or iron-cobalt alloys in gases containing carbon monoxide. Carbon formation is catalyzed by iron, nickel, or cobalt, and the effect is to produce a surface dust layer consisting of a mixture of metal, oxides, and carbon. This dusting is usually observed as pitting or general corrosion attack. Theoretically, alloys that form protective films of the oxides of chromium, silicon, or aluminum should be more resistant. Virtually all hightemperature alloys can be prone to this attack, but higher steam/CO levels help, as does coating with aluminum. In alloys 601, 625 (N06601, N06625), or similar alloys, the attack is tolerable in normal operation.13 Hydrogen sulfide in the gas offers some protection from metal dusting since the adsorbed sulfur blocks the surface for the adsorption of CO or CH4 and other hydrocarbons and the molecules cannot adsorb and dissociate if their adsorption sites are occupied by sulfur. Similarly, dense oxide scales can prevent ingress of carbon into the structure. If sulfur cannot be tolerated in the process, nickel alloys with high chromium and aluminum or silicon additions are the best materials to resist metal dusting.25 Alloy resistance to metal dusting is dependent on its ability to form a protective chromium-oxide scale and can be ranked according to its chromium equivalence: 36 Materials Selector for Hazardous Chemicals Chromium equivalent ⳱ Cr% Ⳮ 3 ⳯ (Si% Ⳮ Al%) (1) Values of chromium equivalent for alloys (some of which are proprietary) commonly used in high-temperature applications in ammonia plants are shown in Table 5.4.23 There are also nickel alloys that are said to have exceptional resistance to metal dusting and elevated-temperature corrosion. The standard alloys, such as alloy 600 and alloy 601, have been used in these environments, but other alloys have been developed with superior high-temperature behavior. Alloy 693 (N06693) was tested for a year in CO and 20% hydrogen at 621⬚C (1150⬚F). Pit depths measured on this alloy were only 0.031 mm, while in the same tests pit depths were 8.164, 3.451, 0.033, 0.34, and 0.293 mm, respectively, on alloy 800 (N08800), alloy DS, alloy 601 (N06601), alloy 620, and alloy 690 (N06690).26 An example of the failure of alloy 601 (N06601) occurred in a heat exchanger on an ammonia plant after only two and a half years of service. It was determined that this failure had been caused by contamination of the process side with steam. This caused mineral deposition that destroyed the protective film within about half a year after the steam ingress into the process side.27 Another nickel alloy developed for resistance to metal dusting and carburizing is alloy 602CA (N06025). The metal wastage rate of this alloy is compared with other nickel alloys in a strongly carburizing CO-H2-H2O gas (see Figure 5.2).28 This alloy has been tested in an ammonia plant in Europe and showed no metal dusting at temperatures of 450⬚C to 850⬚C (842⬚F–1562⬚F). Alloy 601 had some attack, and alloy 800H was severely attacked. There are also coatings being developed to resist metal dusting. Diffusion coatings based on oxide formers such as silicon, titanium, chromium, and aluminum have Table 5.4 Chromium Equivalents for Alloys Commonly Used in Ammonia Plants Nominal % Alloy Wrought alloys 304 800/800H 803 310 600 601 617 214 APM Cast alloys HK-40 HP-Mod XTM Cr Ni Si Al Cr Equiv. Expected Performance 18 20 25 25 15 22 22 16 22 10 32 35 20 72 60 52 76 — — 0.3 0.3 0.3 — — — — — — 0.3 0.3 — — 1.5 1.2 4.5 6.0 18 22 27 26 15 27 26 30 440 Poor Poor Fair Fair Fair Good Good Good Best 25 26 35 20 35 48 1.0 1.5 1.5 — — — 28 30 40 Good Good Best MS-6: Ammonia and Caustic Soda Figure 5.2 37 Metal Wastage Rates of Nickel Alloys in a Strongly Carburizing Atmosphere at Elevated Temperature been tested and found to show good potential to extend the lifetime of iron- and nickel-based alloys under metal dusting conditions.29 Nickel and Its Alloys The nickel alloys are seldom used in ammonia service except at elevated temperatures. They are very resistant to dry ammonia but can be attacked by gaseous ammonia if more than about 1% water is present. They are resistant to anhydrous ammonia and exhibit good resistance to nitriding. The resistance to nitriding in hot, flowing ammonia of various nickel alloys is compared with standard stainless steels in Table 5.5.30 Further data in Table 5.6. show the effect of temperature on nitrogen absorption and nitriding depth for nickel alloys compared with a type 310 (S31000) stainless steel.31 Samples were exposed to pure ammonia for 168 hours. Long-term nitriding tests on alloy 800H/800HT compared with standard stainless steels showed the increased resistance of the high-nickel alloy (see Table 5.7).32 Test conditions were 65% hydrogen, 35% nitrogen, at 11,000 psi (75.8 MPa) and 1000⬚F (540⬚C). Nickel-chromium alloys with 50% to 80% nickel resist pure NH3 to about 500⬚C (932⬚F). Nickel is attacked by wet ammonia in the presence of air in a manner analogous to the reaction with copper. 38 Materials Selector for Hazardous Chemicals Table 5.5 Nitrogen Absorption in Nickel Alloys Exposed to Flowing Ammonia at 1200⬚F (648⬚C) Alloy Nitrogen Absorption (mg/cm2) 230 600 S X 800H 310 304 0.7 0.8 1.3 1.7 4.3 7.4 9.8 Table 5.6 Effect of Temperature on Nitriding Depth in Various Nickel Alloys Nitrogen Absorption (mg/cm2) Nitriding Depth (lm) Mils Alloy 1200⬚F (650⬚C) 1800⬚F (980⬚C) 2000⬚F (1090⬚C) 1800⬚F (980⬚C) 2000⬚F (1090⬚C) 214 230 617 601 X 556 800H 310 1.5 0.7 1.3 1.1 1.7 4.9 4.3 7.4 0.3 1.4 1.5 1.2 3.2 6.7 4.0 7.7 0.2 1.5 1.9 2.6 3.7 4.2 5.5 9.5 1.4 (35.6) 4.9 (124) 15.0 (381) 6.6 (168) 7.4 (188) 14.7 (373) 11.1 (282) 15.1 (384) 0.7 (18) 15.3 (389) ⬎22 (559) ⬎23 (584) ⬎23 (584) ⬎20 (508) ⬎30 (762) ⬎31 (787) Table 5.7 Nitriding Tests in an Ammonia Converter Depth of Nitriding (mm [in.]) Alloy 800H/800HT 310 stainless steel 309 stainless steel 446 stainless steel 304 stainless steel 1-Year Exposure 0.137 0.224 0.241 1.059 1.085 (0.0054) (0.0088) (0.0095) (0.0417) (0.0427) 3-Year Exposure 0.135 0.234 0.244 1.151 1.118 (0.0053) (0.0092) (0.0096) (0.0453) (0.0440) MS-6: Ammonia and Caustic Soda 39 Nickel alloy 200 (N02200) will resist ammonium hydroxide only up to about 1% concentration. Dissolved oxygen may maintain passivation up to about 10% concentration. Higher concentrations are highly corrosive to nickel even in the presence of air (see Table 5.8).33 The corrosion rate of alloy 200 (N02200) in agitated solutions of various strengths of ammonium hydroxide at room temperature is shown in Table 5.9.34 These data show that with agitation, nickel is attacked by moderate concentrations of ammonium hydroxide even at room temperature. Alloy 400, with about 30% copper content, is more resistant than alloy 200 as shown in Table 5.10.34 In solutions of ⬎3% ammonium hydroxide, corrosion rate is increased considerably by aeration and agitation. Table 5.8 Corrosion of Alloy 200 in 1 N Ammonium Hydroxide (1.7% NH3) Test Conditions Total immersion Quiet Air agitated Alternate Immersion Continuous Intermittent Spray—4–30 days Corrosion Rate (mpy [mm/y]) 0.8 (0.020) ⬍0.1 (⬍0.001) 2.7 (0.069) 0.4 (0.01) ⬍0.1 (⬍0.001) Table 5.9 Corrosion of Alloy 200 in Agitated Ammonium Hydroxide Solutions at Room Temperature NH4OH Concentration (%) 1.1 12.9 20.2 27.1 Corrosion Rate (mm/y [mpy]) 0 14.2 (560) 9.4 (370) 4.6 (180) Table 5.10 Corrosion of Alloy 400 in Agitated Ammonium Hydroxide Solutions at Room Temperature NH4OH Concentration (%) 2.7 3.6 5.5 8.2 11.1 18.3 25.8 Corrosion Rate (mm/y [mpy]) 0 1.8 7.6 8.1 8.3 5.9 0.9 (70.9) (299) (319) (327) (232) (35.4) 40 Materials Selector for Hazardous Chemicals The corrosion rate of alloy 800 in 5% and 10% ammonium hydroxide at 80⬚C (176⬚F) was ⬍0.003 mpy (⬍0.1 mpy) in 7-day laboratory tests.32 The nickel-chromium-molybdenum alloys such as N06625, N10276, and so on are resistant but find no application because of the adequate resistance of lesser alloys. Copper and Its Alloys Copper alloys are generally to be avoided in ammonia service. Although resistant to pure, dry NH3, contamination by water and oxygen will cause SCC and general corrosion. Corrosion of various copper alloys in deaerated ammonia is shown in Figure 5.3. and is compared with the behavior of the same alloys in aerated ammonia in Figure 5.4.35 These data clearly show the corrosive effect of the presence of air in the solution. Carbon steel (A-285) is included in these data for comparison, and its corrosion behavior is also adversely affected by the presence of oxygen at lower oxygen levels. Ammonia and copper typically react to form an intensely blue copper/ammonium complex. All copper-based alloys can be made to crack in ammonia vapor, ammonia solutions, ammonium ion solutions, and environments in which ammonia is formed. It is generally true that any metal with a small grain size is more resistant Figure 5.3 Corrosion of Various Copper Alloys in Deaerated Ammonia MS-6: Ammonia and Caustic Soda Figure 5.4 41 Corrosion of Various Copper Alloys in Aerated Ammonia to SCC regardless of whether the cracking is transgranular or intergranular. The effect of grain size on the time to cracking of yellow brass (C26800) in ammonia is shown in Figure 5.5.36 While some copper-based alloys are far superior to others in their resistance and dry anaerobic ammonia does not cause corrosion, it is general industry practice to avoid all use of copper-based alloys in ammonia and related services. It can be noted in passing that copper in solid solution in ferrous metals (generally less than 3%) added to attain certain physical, mechanical, or corrosion properties does not pose a problem in ammonia applications. Copper alloys C11200 and C26000 were penetrated at a rate of 5 lm/y (0.2 mpy) in anhydrous ammonia at atmospheric temperature and pressure. Corrosion rates were also low if small amounts of water were present, but oxygen was also probably excluded.2 Copper alloy tubes in utility condensers have often been attacked by ammonia on the steam side. The ammonia in this case comes from decomposition of amines or hydrazine added to the boiler feed to control oxygen and passivate the boiler surface. Admiralty brass is commonly used to tube such condensers and has been subject to this attack by ammonia. Copper alloy condensers form a surface layer of cuprous oxide when placed in surface. If excess oxygen is present, this oxide layer is converted to cupric oxide, which is readily complexed by ammonia. Attack by ammonia in this type of utility condenser is accelerated by air in-leakage. In cases where boiler chemistry cannot be altered or controlled to avoid ammonia formation, a more resistant alloy, such as copper-nickel, is used.37,38 42 Materials Selector for Hazardous Chemicals Figure 5.5 The Effect of Grain Size on the Time to Cracking of Yellow Brass (C26800) in Ammonia All copper-based alloys are attacked by ammonium hydroxide unless air is rigorously excluded, which is not feasible in plant practice. The deep royal blue of the ammonium/copper complex is immediately obvious. Dealloying can also occur in some copper alloys in ammonia solutions. It was found that dezincification occurred in 70/30 brass in 10 N ammonium hydroxide solution at 32⬚C (90⬚F). Corrosion and dezincification were increased by the application of increasing stress to the alloy. The mechanism of this increased attack was shown to be due to an increase in open-circuit potential and a shift in the polarization curve under the influence of applied stress.39 Titanium and Its Alloys Titanium in not attacked by atmospheres containing ammonia but can be corroded at elevated temperatures. The protective oxide film is effective in ammonia up to at least 300⬚C (572⬚F).2 At higher temperatures, ammonia will decompose into nitrogen and hydrogen that may cause hydrogen embrittlement of titanium. Titanium corroded at 440 mpy (11.2 mpy) in an ammonia-steam mixture at 431⬚F [221⬚C]). This high corrosion rate was thought to be associated with hydriding. Titanium shows excellent resistance to corrosion in up to 70% ammonium hydroxide up to the boiling point.40 The corrosion rate of titanium in 100% anhydrous ammonia is ⬍0.13 mm/y at 40⬚C (104⬚F). In 28% ammonium hydroxide solution at room temperature, the rate is 0.0025 mm/y.41 MS-6: Ammonia and Caustic Soda 43 Zirconium and Its Alloys Zirconium is resistant to ammonia even at elevated temperatures. The corrosion rate of zirconium Zr702 (R60702) in wet ammonia at 38⬚C (100⬚F) is less than 5 mpy (⬍0.127 mm/y) and in 28% ammonium hydroxide at up to 100⬚C (212⬚F) is ⬍1 mpy (⬍0.025 mm/y).42 Zirconium is stable in ammonia up to about 1000⬚C (1832⬚F).2 Niobium Niobium is not attacked by 13% and 25% ammonium hydroxide solutions at 20⬚C to 100⬚C (68⬚F–212⬚F).1 Tantalum Tantalum is resistant to anhydrous liquid ammonia but should not be exposed to the gaseous mixtures encountered in ammonia synthesis at elevated temperature. Above about 250⬚C (482⬚F), it reacts rapidly with hydrogen to form brittle hydrides. Tantalum is not corroded by 10% aqueous ammonium hydroxide solutions up to 100⬚C (212⬚F) but is attacked by hot, concentrated ammonium hydroxide solutions.1 Other Metals and Alloys Lead is resistant in ammonia at temperatures up to 60⬚C (140⬚F), and hard lead is satisfactory up to 100⬚C (212⬚F) in dry ammonia. Lead is also resistant to ammonium hydroxide at room temperature.43 The corrosion rate is very sensitive to the presence of air and agitation. In 27% ammonia solution at 20⬚C (68⬚F), lead had a corrosion rate of 110 g/m2/d (3.3 mm/y) with rapid agitation and only 21 g/m2/d (0.63 mm/y) without agitation.5 Tin is resistant to dry ammonia and saturated ammonia solutions, but diluted ammonia solutions corrode tin. Zinc is not resistant to ammonium hydroxide. Magnesium is not attacked by wet or dry ammonia at ordinary temperatures, but attack may occur if water vapor is present. The precious metals are also not used. In fact, there is a potential hazard if silver is exposed under some conditions because explosive azides may be formed. Precious metals are not employed, and silver or silver-rich alloys are not to be employed in ammonia or ammonium hydroxide. 44 Materials Selector for Hazardous Chemicals References 1. Anon, Dechema Corrosion Handbook, Ammonia and Ammonium Hydroxide section, CD-ROM (Frankfurt, Germany: Dechema aV, 2001). 2. B.D. Craig, D.B. Anderson, eds., Handbook of Corrosion Data (Materials Park, OH: ASM International, 1997), pp. 128–135. 3. Anon, Aluminium with Food and Chemicals (London, UK: Alcan Industries Ltd, 1966), p. 18. 4. F.L. LaQue, H.R. Copson, Corrosion Resistance of Metals and Alloys, 2nd ed. (New York, NY: Reinhold Publishing Corp., 1963), 365 pp. 5. E. Rabald, Corrosion Guide (Amsterdam, Netherlands: Elsevier Scientific Publishing Co., 1968), pp. 46–50. 6. R. Covert, J. Morrison, K. Rohrig, W. Spear, Ni-Resist and Ductile Ni-Resist Alloys, reference book no. 11018 (Toronto, ON, Canada: NiDI, 1998), 42 pp. 7. P. Ludwigsen, H. Arup, “Stress Corrosion Cracking of Mild Steel in Ammonia Vapor above Liquid Ammonia,” Corrosion 32, 11(1976): pp. 430–431. 8. A.W. Loginow, “Stress-Corrosion Cracking of Steel in Liquefied Ammonia Service,” MP 25,12 (1986): pp. 18–22. 9. L. Lunde, R. Nyborg, “Stress Corrosion Cracking of Carbon Steel Storage Tanks for Anhydrous Ammonia,” Proc. International Fertiliser Society—Proceeding 307 (1991). 10. P. McGowan, “Managing Ammonia Stress Corrosion Cracking,” Plenary Address to the Australasian Corrosion Association Conference (Auckland, New Zealand: Australasian Corrosion Association, 2000). 11. M.M. Ghanem, A.M. Elbatahgy, “Catastrophic Failure of Liquefied Ammonia Gas Cylinder,” MP 42, 4 (2003): pp. 52–55. 12. K.C. Pattnaik, M.P. Gupta, “Stress Corrosion Cracking of Ammonia Receiver Tank,” Br. Corr. J. 30, 1 (1995): p. 80. 13. M. Appl, Ammonia: Principles and Industrial Practice (Weinheim, Germany: Wiley-VCH, 1999), pp. 209–221. 14. K.L. Baumert, G.V. Krishna, D.P. Bucci, “Hydrogen Attack of Carbon-0.5 Molybdenum Piping in Ammonia Synthesis,” MP 25, 7 (1986): pp. 34–37. 15. API 941, “Steels for Hydrogen Service at Elevated Temperatures and Pressures in Petroleum Refineries and Petrochemical Plants” (Washington, DC: API, latest ed.). 16. L.P. Antalffy, G.T. West, “The New Generation Vanadium Modified Steels,” minutes of EFC WP15 meeting, Total Fina Elf (Paris, France, 2002). 17. Anon, “AL 29-4C,” Technical Data Sheet B-151-Ed5/7.5M/793/GP (Pittsburgh, PA: Allegheny Ludlum Steel Corporation, 1993), 8 pp. 18. G. Kobrin, J. Lilly, J. Mac Diarmid, B. Moniz, “Stainless Steels for Chemical Process Equipment,” NiDI reprint series no. 14 038 (Toronto, ON, Canada: NiDI, 1998), pp. 1–9. 19. J.M. Stone, “Corrosion Resistance of Nickel and Nickel-Containing Alloys in Caustic Soda and Other Alkalies,” CEB-2 (New York, NY: International Nickel Company Inc., 1973), p. 20. MS-6: Ammonia and Caustic Soda 45 20. S.K. Bhaumik, R. Rangaraju, M.A. Parameswara, T.A. Bhaskaran, M.A. Venkataswamy, A.C. Raghuram, R.V. Krishnan, “Failure of Reformer Tube of an Ammonia Plant,” Engineering Failure Analysis 9 (2002): pp. 553–561. 21. K.M. Verma, H. Ghosh, K.C. Pattnaik, “Stress Corrosion Failure of Waste Heat Boiler Tubes in an Ammonia Synthesis Converter,” Br. Corr. J. 15, 4 (1980): pp. 175–178. 22. M.P. Sukumaran Nair, “Tackling Corrosion in Ammonia Plants—Selecting the Proper Materials,” Chemical Processing 12, 1 (2001). 23. S.B. Parks, C.M. Schillmoller, “Improve Alloy Selection for Ammonia Furnaces,” Hydrocarbon Processing (Int. ed.) 76, 10 (1997): pp. 93–98. 24. O. de Sanctis, L. Gomez, N. Pellegri, A. Durfi, “Behaviour in Hot Ammonia Atmosphere of Sio2-Coated Stainless Steels Produced by a Sol-Gel Procedure,” Surface and Coatings Technology 70 (1995): pp. 251–255. 25. H.J. Grabke, “Metal Dusting,” Materials and Corrosion 54, 10 (2003): pp. 736– 746. 26. S. McCoy, “Inconel Alloy 693 Exceptional Metal Dusting and High Temperature Corrosion Resistance,” minutes of EFC WP15 meeting, Total Fina Elf (Paris, France, 2002). 27. H.J. Grabke, M. Spiegel, “Occurrence of Metal Dusting—Referring to Failure Cases,” Materials and Corrosion 54, 10 (2003): pp. 799–804. 28. D.C. Agarwal, L. Stewart, M. McAllister, “Alloy 602CA (UNS N06025) Solves Pig Tail Corrosion Problems in Refineries,” CORROSION/2003, paper no. 03495 (Houston, TX: NACE International), 17 pp. 29. C. Rosado, M. Schutze, “Protective Behaviour of Newly Developed Coatings against Metal Dusting,” Materials and Corrosion 54, 11 (2003): pp. 831–853. 30. Anon, “Haynes 230 Alloy for Industrial Heating Applications—Data Summary,” Brochure H-3033G (Kokomo, IN: Haynes International, 2002), p. 19. 31. F.G. Hodge, “High Performance Alloys Solve Problems in the Process Industries,” CORROSION/91, paper no. 173 (Houston, TX: NACE, 1991), p. 9. 32. Anon, “Solutions to Materials Problems,” CD-ROM (Huntington, WV: Inco Alloys International, 1997). 33. Anon, “Corrosion Resistance of Nickel and Nickel-Containing Alloys in Caustic Soda and Other Alkalies,” CEB-2 (New York, NY: International Nickel Company Inc., 1973), p. 21. 34. Anon, “Corrosion Resistance of Nickel and Nickel-Containing Alloys in Caustic Soda and Other Alkalies,” CEB-2 (New York, NY: International Nickel Company Inc., 1973), p. 22. 35. N.W. Polan, et al. (1981), in Metals Handbook—Corrosion, vol. 13, 9th ed., ed. J.R. Davis (Metals Park, OH: ASM International, 1987), p. 622. 36. H.H. Uhlig (1971), in Metals Handbook—Failure Analysis and Prevention, vol. 11, ed. G.W. Powell, S.A. Mahmoud (Metals Park, OH: ASM International, 1986), p. 208. 37. B.J. Buecker, “Watch Out for Steam-Side Corrosion in Utility Condensers,” MP 31, 9 (1992): pp. 68–70. 38. B.J. Buecker, E. Loper, “Steam Surface Condenser Tubes: Watch Out for Sneaky Corrosion,” MP 39, 5 (2000): pp. 60–64. 46 Materials Selector for Hazardous Chemicals 39. T.K.G. Namboodhiri, R.S. Tripathi, “The Stress-Assisted Dezincification of 70/ 30 Brass in Ammonia,” Corrosion Science 26, 10 (1986): pp. 745–756. 40. Anon, “Corrosion Resistance of Titanium,” brochure no. TMC-0105 (Denver, CO: Timet, 1999), p. 22. 41. Anon, “Corrosion Resistance of Titanium,” ref. no. 1431531969 (Birmingham, UK: IMI Kynoch Ltd, 1969), pp. 28, 36. 42. Anon, “Zircadyne Corrosion Data,” bulletin no. TWCA-8101ZR (Albany, OR: Teledyne Wah Chang, 1987), 25 pp. 43. Anon, “Corrosion of Lead” (London, UK: Lead Development Association, 1971). 6 Resistance of Nonmetallic Materials Nonmetallic materials (i.e. elastomers, plastics, ceramics, and carbon products) find few applications in ammonia systems except occasionally as O-rings and gaskets. Some nonmetallic materials do, however, find limited applications in ammonium hydroxide service. Elastomers Some elastomeric materials perform well in strong bases such as ammonia. Satisfactory materials include perfluoroelastomers, special grades of fluorohydrocarbon elastomers (e.g. Viton威TBR-S), polychloroprene rubber, acrylonitrile rubber (BunaN威), and butadiene-styrene rubber (Buna-S威). Ethylene propylene diene monomer rubber (EPDM) is resistant to ammonia but may be attacked by oils present in compressed gas systems. Buna-N威 elastomer are suitable for cold ammonia gas, but their performance is only fair to poor in hot gas. Neoprene is suitable in cold ammonia while PTFE is acceptable across the temperature range in ammonia.1 Satisfactory materials in ammonium hydroxide include perfluoroelastomers and polychloroprene rubber. Some elastomers that are attacked and are unsatisfactory are butyl, general purpose FKM, hard rubber, isoprene, and natural rubber. Temperature limits for various elastomers are given in Table 6.1. These data are a compilation from various sources. Testing is recommended for applications involving elastomers in specific ammonia environments. Plastics Most plastics are chemically resistant to ammonia and ammonium hydroxide at ambient temperatures. However, because of the hazardous nature of ammonia, the 47 48 Materials Selector for Hazardous Chemicals Table 6.1 Estimated Temperature Limits (⬚C [⬚F]) for Various Elastomers That May Be Suitable in Ammonia Elastomer Family Saturated Gaseous Liquid Ammonia Ammonia Ammonia Solution Chloroprene (CR, neoprene) Chlorosulfonated polyethylene (CSM, Hypalon威, etc.) Ethylene propylene (EPR, EPDM, Nordel威, etc.) Fluoroelastomer (FKM, Viton威 TBR-S, ETP-S) Natural rubber (NR) Nitrile rubber (NBR, Buna-N威, etc.) Perfluoroelastomers (FFKM, Kalrez威 6375, 1050LF, etc.) 60 (140) 20 (68) 80 (176) 60 (140) 20 (68) — 60 (140) 20 (68) 80 (176) 50 (122) 50 (122) 50 (122) 60 (140) 20 (68) 60 (140) 40 (104) 20 (68) 60 (140) 260 (500) 240 (464) 260 (500) use of plastics is generally not recommended except in seals and gaskets, where fluoropolymers are typically used. Thermoplastics Resistant grades of fluoropolymers are ECTFE (Halar威), ETFE (Tefzel威), PVDF (Kynar威), FEP, PFA, and PTFE (Teflon威). Some plastics that are generally unsatisfactory are ABS, epoxy, certain polyesters, polyisobutylene, and polystyrene. Data on temperature limits for various common thermoplastics are shown in Table 6.2. These data are a compilation from various sources. Various manufacturers list elevated temperature limits for plastics in liquid ammonia, and these are included in this table. They are not, however, of very practical interest since it is highly unlikely that plastics would be used in elevated temperature (therefore, also high pressure) liquid ammonia. In the unlikely event that an application for a plastic was being Table 6.2 Suggested Temperature Limits (⬚C [⬚F]) for Various Plastics in Ammonia Plastic Gaseous Ammonia Liquid Ammonia 10% Ammonia Solution Polyethylene (PE) Polybutylene (PB) Polypropylene (PP) Polyvinyl chloride (PVC) Chlorinated PVC (CPVC) Polyvinylidene fluoride (PVDF) Polytetrafluoro ethylene (PTFE) 60 (140) 60 (140) 60 (140) 60 (140) 40 (104) fair 80 (176) 120 (248) 60 (140) 20 (68) 60 (140) 60 (140) — 40 (104) 120 (248) 60 (140) 60 (140) 60 (140) 60 (140) 40 (104) fair 100 (100) 120 (248) MS-6: Ammonia and Caustic Soda 49 considered in liquid ammonia, the subzero properties would be more relevant and should be investigated. One area of interest is the difference in behavior of PVC and CPVC. In many environments, CPVC is more resistant than PVC and can withstand higher temperatures. This is not the case, however, in ammonia and amines. PVC has generally good resistance to ammonia and some amines, even at somewhat elevated temperatures, while CPVC has extremely poor resistance to ammonia or ammonium hydroxide and limited resistance to most amines, even at ambient temperatures. This is due to the extremely high reactivity of amines and chlorine, the higher availability of chlorine on the CPVC, and its lower bond strength on CPVC versus PVC. Even at fairly low concentrations and temperatures, ammonia and many amines are capable of rapid dehydrochlorination of CPVC. One CPVC pipe handling 28% ammonium hydroxide at ambient temperature failed after only one year in service.2 If polyethylene is used to store concentrated aqueous ammonium solutions, there is a weight loss due to outward diffusion through the plastic. A solution of 27% ammonium hydroxide solution kept in a 500-cm3 bottle, 1-mm wall at 20⬚C (68⬚F) for 54 days lost 3.6% of its weight.1 Use of many of these thermoplastics is confined to linings for pipe and vessels rather than as solid construction items because of the hazardous nature of ammonia. Recommended temperature limits for plastic-lined steel pipes are given in Table 6.3.3 Thermoplastics are also used as the resistant liner in dual laminate construction in which fiber-reinforced plastic (FRP) is used as the reinforcing, structural element. The corrosion resistance depends on the resistance of the thermoplastic liner, although resistant resins are often used in the FRP reinforcement in case of permeation and leaks in the thermoplastic liner. Most common thermoplastics are used in this type of construction, and many of them are suitable for use in ammonia applications (see Table 6.4).4 Table 6.3 Recommended Temperature Limits for Plastic-Lined Pipe in Ammonia Ammonia or Hydroxide Anhydrous gas Anhydrous liquid 1% ammonium hydroxide 10% ammonium hydroxide Concentrated Ammonium hydroxide PP PVDF PTFE 150⬚F (65⬚C) 225⬚F (110⬚C) 225⬚F (110⬚C) 225⬚F (110⬚C) 225⬚F (110⬚C) NR NR 225⬚F (110⬚C) 225⬚F (110⬚C) 225⬚F (110⬚C) 450⬚F (230⬚C) 450⬚F (230⬚C) 450⬚F (230⬚C) 450⬚F (230⬚C) 450⬚F (230⬚C) Note: NR ⳱ not recommended Thermoset Resins The fiberglass-reinforced thermoset composites, commonly called FRP, have a resistance determined by the polymer used. Some suggested concentration and tem- 50 Materials Selector for Hazardous Chemicals Table 6.4 Suggested Temperature Limits (⬚C [⬚F]) for Various Plastics in Dual Laminate Construction in Ammonia Plastic Gaseous Ammonia (Technically Pure) Aqueous Ammonium Hydroxide (⬃25%) PVC CPVC (chlorinated PVC) PE PP PVDF ECTFE (ethylene chlorotrifluoroethylene) ETFE (ethylene trifluoroethylene) FEP (fluorinated ethylene propylene) PFA (perfluoro alkoxy) 60 (140) — 60 (140) 60 (140) 40 (104), 100* (212) 20 (68) 40 (104), 60* (140) 40* (104) 60 (140) 60 (140) — 100 (212) 150 (302) 150 (302) 150 (302) 150 (302) 150 (302) 150 (302) *Conditionally resistant at this temperature. The medium can attack the material or cause swelling. Restrictions must be made in regard to pressure and/or temperature, taking the expected service life into account. The service life of the installation can be noticeably shortened.4 perature limitations of FRP are as shown in Table 6.5. Data are a compilation from various sources. Standard FRP piping is also available in a number of different resins and types of construction. The recommended limits of application of one commercial supplier are shown in Table 6.6.5,6 Some of these pipes are cast, and others are filament wound, depending on the resin system and application. Table 6.5 Suggested Temperature Limits for FRP in Ammonia Service Ammonia or Ammonium Hydroxide Bisphenol A Fumurate Vinyl Ester Epoxy Furane Liquefied ammonia Ammonia gas, dry Ammonia gas, wet 5% ammonium hydroxide 10% ammonium hydroxide 20% ammonium hydroxide 29% ammonium hydroxide NR 140⬚F (60⬚C) 200⬚F (93⬚C) 180⬚F (82⬚C) 140⬚F (60⬚C) 140⬚F (60⬚C) 100⬚F (38⬚C) NR 200⬚F (93⬚C) 200⬚F (93⬚C) 180⬚F (82⬚C) 140⬚F (60⬚C) 140⬚F (60⬚C) 100⬚F (38⬚C) NR — — 180⬚F (82⬚C) 160⬚F (71 oC) 151⬚F (66⬚C) 126⬚F (52 oC) NR — — 100⬚F (38⬚C) 100⬚F (38⬚C) NR NR Note: NR ⳱ not recommended *Limiting allowable concentration 150 (66) — NR 120 (49) 120 (49) 100 (37.8) 100 (37.8) — Ammonia Dry, anhydrous gas2,4 Wet gas Liquid 5% solution 10% solution 20% solution 28% solution Saturated solution 225 (107) — NR 150 (66) 150 (66) 125 (52) 125 (52) — Epoxy (Green Thread) 100 (37.8) — NR 180 (82) 165 (74) 150 (66) 125 (52) — Chem Thread 275 (135) — NR 200 (93) 200 (93) 200 (93) 200 (93) 175 (79) Proprietary (Z-Core) 150 (66)1 150 (66)1 NR 150 (66) 150 (66) 150 (66) 100 (37.8) — Epoxy (RB-2530, RB- 1520) 100 (37.8) 100 (37.8) NR 150 (66) 150 (66) 150 (66) 100 (37.8) — Vinyl ester (CL-2030) Proprietary (CL-1520) 100 (37.8)1 100 (37.8) NR 1803,5 150 (66)3,5 150 (66)3,5 100 (37.8)3,5 — Various Resins (F-CHEM6 F-CHEM-V) Note: NR ⳱ not recommended 1. Maximum temperature for which information is available; could be serviceable at higher temperatures. 2. Dry gases under pressure can condense to liquids in cool weather. This situation should be avoided. 3. A double synthetic veil liner is recommended. 4. Pipeline for all pressurized gas applications should be buried at least 3 feet deep. Not recommended for above ground pressurized gas lines if the operating pressures exceed 25 psig for 1- to 6-inch pipe, 14 psig for 8-inch pipe, 9 psig for 10-inch pipe, 6 psig for 12-inch pipe, 5 psig for 14inch pipe, 4 psig for 16-inch pipe, and 1 psig for 18-inch and larger sizes. 5. A bisphenol vinyl ester or epoxy resin is preferred for this application. 6. Based on standard bisphenol A vinyl ester resin. Epoxy (Red Thread II) Table 6.6 Estimated Temperature Limits ⬚F (⬚C) of Commercial FRP Piping MS-6: Ammonia and Caustic Soda 51 52 Materials Selector for Hazardous Chemicals Carbon and Graphite Carbon and graphite are resistant to ammonium hydroxide. However, with impervious graphite heat exchangers, an epoxy resin should be specified rather than phenolic, which is attacked by alkaline chemicals. Carbon and graphite are resistant to all solutions of ammonia up to their limiting temperature, which depends on the individual grade and formulation. Graphite is resistant to anhydrous ammonia over the full range of concentration up to the temperature limit of the graphite. Fluorocarbon-bonded graphite, Diabon F100威, is resistant in 20% ammonia/caustic ammonia at up to 40⬚C (104⬚F).7 Ceramic Materials Glass is resistant to about 60⬚C (140⬚F) in very dilute solutions of ammonium hydroxide (perhaps 1%) but will withstand solutions to pH 14 at room temperature. See Figure 6.1 for more details on rate of attack on glass-lined steel.8 Figure 6.1 Wastage Rates (in mm/y) of Glass-Lined Steel in Ammonia. Vol: Surface Area ⳱ 20 MS-6: Ammonia and Caustic Soda 53 References 1. Anon, “Corrosion Resistance Guide” (Chicago, IL: Yamada Pumps Inc., 2001), p. 6. 2. M.L. Knight, “Failure Analysis Of PVC and COVC Piping Materials,” CORROSION/2003, paper no. 03606 (Houston, TX: NACE International), 7 pp. 3. Anon, “Chemical Resistance Guide” (Bay City, MI: Dow Chemical, 1991), 20 pp. 4. Anon, “Chemical Resistance of Thermoplastics Used in Dual Laminate Constructions,” DLFA (2002), 143 pp., http://www.dual-laminate.org/html/ corrosion_guide.html. 5. Anon, “Chemical Resistance Guide,” bulletin no. E5615 (Little Rock, AR: Smith Fibercast, 2003), 16 pp. 6. Anon, “Smith Fibercast FRP Pipe & Fittings” (Sunshine, LA: CorPro Inc., 2003). 7. Anon, “The Chemical Industry Builds on Graphite,” brochure no. PE 200/07 (Meitingen, Germany: SGL Carbon Group, 2001), 24 pp. 8. Anon, “Worldwide GLASTEEL 9100,” brochure no. SB95-910-5 (Rochester, NY: Pfaudler Reactor Systems, 2000), p. 5. 7 Corrosion in Contaminated Ammonia There are various kinds and degrees of contamination encountered in ammonia production and usage, and these may profoundly affect the corrosion behavior of the materials of construction commonly used in this service. As mentioned in Chapter 3, there are some impurities inherent in the manufacturing process that are substantially removed during the refining process. In other instances, the ammonia becomes contaminated with other impurities or may be mixed with other chemicals deliberately for specific purposes. The presence of specific contaminants in ammonia and ammonium hydroxide streams, other than the oxygen-induced stress corrosion cracking and water inhibition previously discussed, can cause unexpected corrosion. Ammonium Chloride Organic chlorides in naphtha are converted to hydrogen chloride (HCl) in the NHT (naphtha hydrotreater), where they combine with ammonia (NH3) to form ammonium chloride (NH4Cl). The amount and rate of NH4Cl formation is controlled by the HCl level because of the overabundance of ammonia in the NHT effluent coming from the conversion of nitrogen in the feed. Depending on the chloride level in the feed, the location of NH4Cl deposition can shift. Solid ammonium chloride tends to deposit at temperatures as high as 150⬚C to 170⬚C, a temperature range often corresponding to the coldest effluent exchangers. While the dry NH4Cl salt is not corrosive, it can cause fouling and plugging of heat exchanger tubes. It is also very hygroscopic, and the wet salt can be extremely corrosive. Most corrosion problems in hydrotreaters result from the deposition of wet ammonium chloride.1 Carbon Dioxide—Carbamates If carbon dioxide, ammonia and large quantities of water are together at ambient temperatures, noncorrosive ammonium carbonate or bicarbonate is formed. At el55 56 Materials Selector for Hazardous Chemicals evated temperatures (⬎60⬚C) at atmospheric pressure or above, carbamates such as ammonium carbamate NH4CO2NH2 (or NH2COONH4) can be formed. These are very corrosive to carbon steel (rates of 3 mm/y [120 mpy]) and to stainless steels at higher temperatures and pressures.2 Ammonium carbamate can hydrate to the noncorrosive ammonium carbonate or thermally decompose back to ammonia and carbon dioxide. These various reactions may compete in different parts of ammonia stripping stills and give very local areas of corrosion. The corrosive carbamate may be transient, depending on variations in CO2 content, temperature, and so on. Ammonium carbamate is also a transient intermediate that exists during the production of urea by the following reactions:3 2NH3 Ⳮ CO2 r NH2COONH4 (ammonium carbamate) Ⳮ heat NH2COONH4 r CO(NH2 )2 (urea) Ⳮ H2O (1) Carbamates can cause severe corrosion in urea production, and oxygen injection has been used to maintain passivity of stainless steels in this process. Other manufacturers have used titanium or zirconium to avoid this type of corrosion. In laboratory tests, the vapor-phase corrosion rate of steel increased by an order of magnitude when CO2 was bubbled through 28% ammonium hydroxide instead of air. Severe corrosion has occurred in localized sections of a stripping column in the production of alkylamines, in which an alcohol and ammonia are reacted catalytically: C2H5OH Ⳮ NH3 r C2H5-NH2 Ⳮ H2O (2) Carbamate corrosion of the steel column and type 410 (S41000) trays occurred in the ammonia column, while feed and tails piping, reflux area and reboiler, and 304 (S30400) trays were unattacked. The aggressive carbamates form only in local areas under specific conditions, so this problem is often very localized and severe. Corrosion of steel ammonia stripping columns has occurred in alkanolamine and ethylene-amine processes. In the former process, replacement of the carbon steel by type 304 (S30400) stainless steel was successful; in the later process, type 316 (S31600) was attacked, and titanium was found to be necessary.4 Extensive corrosion was found within six months of startup of type 304 (S30403) components in a intercooler and aftercooler of a CO2 gas cleaning circuit in a urea plant. The failure of the shell, fins, demisters, and sealing strips was identified as being due to corrosion by CO2 and/or the reaction product of CO2, ammonia, and water vapor (probably ammonium carbamate). This attack was aided by the galvanic effects between the type 304 (S30400) stainless steel and the 3RE60 (S31500) duplex stainless steel tubes. The use of type 304 for all components and the addition of oxygen to the CO2 have been recommended to avoid this problem with the replacement exchangers.5 Contamination problems with ammonium hydroxide are rare, although it will absorb carbon dioxide from air to form ammonium carbonate. Usually, aqueous solutions do not see high enough temperatures to cause significant corrosion via the MS-6: Ammonia and Caustic Soda 57 carbamate mechanism. However, steel coupons in the vapor phase over 28% ammonium hydroxide corroded at about 20 mpy when a stream of carbon dioxide was passed through the vapor space. This test indicated formation and replenishment of ammonium carbamate corrosion.6 Chlorides Chloride contamination in ammonium hydroxide does not cause increased corrosion of ferrous alloys or even significant pitting or crevice corrosion of stainless steels. This is presumably due to the high pH of the solution. However, reactions can occur to produce traces of corrosive species that will cause corrosion problems if constantly replenished. References 1. S. Kapusta, F. van den Berg, R. Daane, M.C. Place, “The Impact of Oil Field Chemicals on Refinery Corrosion Problems,” CORROSION/2003, paper no. 03649 (Houston, TX: NACE International), 12 pp. 2. C.P. Dillon, Corrosion Control in the Chemical Process Industries, 2nd ed. (St. Louis, MO: MTI, 1994), p. 287. 3. Anon, “Production of Urea and Urea Ammonium Nitrate,” vol. 5 (Brussels, Belgium: EFMA, European Fertilizer Manufacturers’ Association, 2000), 44 pp. 4. C.P. Dillon, “Carbamates Can Cause Corrosion Problems,” MP 38, 12 (1999): pp. 74–75. 5. H. Shaikh, R.V. Subba Rao, R.P. George, T. Anita, H.S. Khatak, “Corrosion Failures of AISI Type 304 Stainless Steel in a Fertiliser Plant,” Engineering Failure Analysis 10 (2003): pp. 329–339. 6. C.P. Dillon, Materials Selection for the Chemical Process Industries (New York, NY: McGraw-Hill Inc., 1991), pp. 325–326. 8 Specific Production Equipment The corrosion resistance of various materials under a variety of conditions has been discussed in previous chapters. Materials of construction that are being or have been used for specific equipment are detailed in this chapter, which also covers aspects of design and operation that are relevant to particular types of equipment. One critical aspect of design is the specification of suitable materials of construction to adequately resist the aggressive environment. Mechanical design is the other essential design step, and the basic requirements are described in several standards, such as API 650 for Storage Tanks and ASME Section VIII, Division 1 for Pressure Vessels.1,2 All pressure vessels should be built and tested to the requirements of the construction code to which they are built. Details on ammonium salts and organic derivatives (amines) are beyond the scope of this monograph. Production Stages Different materials are used at various stages of ammonia production. A modern ammonia plant is shown in Figure 8.1, and a schematic production flow sheet is shown in Figure 8.2. The items of equipment can conveniently be discussed under the relevant process stages. Desulfurization Section Stainless steel types S30400, S31600, or S32100 are often used in the construction of equipment items in this part of the process to resist high-temperature attack by hydrogen sulfide and carbonyl sulfide. External corrosion and thinning of fired heater coils and interior deposition of carbon resulting from coking (leading to overheating) can cause failures. Fuel gas lines containing hydrocarbon vapors and hydrogen sulfide must be of type 304 (S30400) material and preferably have heat tracing to avoid condensation.3 59 60 Materials Selector for Hazardous Chemicals Figure 8.1 Part of a Modern Ammonia Plant Using Coal as a Feedstock (photo courtesy of Sasol Ltd, Secunda, RSA) Primary Reformer Steam-hydrocarbon reforming over a nickel catalyst usually takes place in a tubular externally fired furnace operating at pressures from 25 to 35 bar and at temperatures from 780⬚C to 820⬚C (1436–1508⬚F). The reformer tube can suffer from carburization, oxidation, overheating, stress-corrosion cracking, sulfidation and thermal cycling. The standard stainless steels, types 304 (S30400), 310 (S31000), and 347 (S34700), have failed by cracking, so a better alloy (HK 40, 25% Cr, 20% Ni) was developed and proved satisfactory for vertical reformer tubes, permitting plant capacity to reach 600 t/d. This material has a design life of 100,000 operating hours, but this is considerably shortened if overheating occurs. If an HK 40 tube is subjected to a service temperature of 55⬚C (100⬚F) above the prescribed base temperature, its service life will be reduced by about 90%, as shown in Figure 8.3.4 HP (chromium, nickel) alloys were modified to have improved creep resistance with good ductility and weldability. The modifications of the HP alloys include Figure 8.2 Ammonia Production Flow Sheet Showing Principal Items of Equipment (courtesy M.P. Sukumaran Nair, FACT Ltd, Cochin, India) MS-6: Ammonia and Caustic Soda 61 62 Materials Selector for Hazardous Chemicals Figure 8.3 Effect on Service Life of Overheating an HK 40 Tube above the Prescribed Base Temperature additions of some combination of molybdenum, niobium, and/or tungsten of which the HP 45Nb is one of the most commonly used. Stress to rupture values for these alloys is compared with the original HK 40 in Figure 8.4.5 These data clearly show the improvements in elevated temperature properties obtained from these alloys. When these stronger alloys are specified, thinner-walled tubes can be used, reducing tube supports and increasing heat transfer. A number of plants all over the world have been revamped with HP-modified tubes in their primary reformers, increasing plant capacities by up to 30%. A more recent development is the production of HP microalloys that have better resistance to carburization and better high-temperature creep-rupture properties.3 Alloys such as 25% Cr, 35% Ni, 15% Co, 5% W; 28% Cr, 48% Ni, 5% W; and 35% Cr, 45% Ni plus additional elements have been developed to offer higher strength and good high-temperature properties. These strong alloys either can provide a higher operating temperature with better product yield or are offered as a substitute for HK-40 or HP-Mod and improve run length and tube life.5 Originally, HK, HT, and HU alloys were used for risers, manifolds, and transfer headers, and many of these failed from thermal cycling during startup and shutdown. Wrought alloy 800H (N08800) was the normal replacement alloy used. Although it is not as strong as the cast alloys, it is more ductile and resistant to thermal shock.5 The cast version of alloy 800H is a cheaper alternative with a higher creeprupture strength, a low tendency for embrittlement, and good ductility but is still rarely used in this application. Hot reformed gas transfer lines usually are refractory lined with an interior alloy 800 sheathing.3 MS-6: Ammonia and Caustic Soda Figure 8.4 63 Stress to Rupture Data for HP Modified Alloys Compared with HK 40 As part of an upgrade at the Ruwais Fertilizer Industries (FERTIL) plant in Abu Dhabi, the primary reformer tubes that were IN-519 (24% Cr, 24% Ni) were replaced with an HP-modified material (KHR 35 CT, Kubota, Japan). The design life of the original tubes was 100,000 hours, and they were replaced in 1998, when they had completed 116,000 operating hours. Because of the improved material characteristics, it was possible to increase the ID of the tubes, which accommodated about 24% extra catalyst and allowed increased throughput. At the same time, the secondary reformer (refractory lined chromium, 1/2% Mo) was replaced by a refractory-lined 11/4% Cr, 1/2% Mo unit.6 Secondary Reformer The secondary reformer operates at 957⬚C to 1025⬚C (1755⬚F–1877⬚F) and is a refractory-lined vessel with an outer shell of a low-alloy (0.5% Mo) steel. Metal dusting can occur at this stage of the process, and type 304 stainless or alloy 800 (N08800) is very susceptible to this type of attack in the temperature range 500⬚C to 800⬚C (932⬚F–1471⬚F). Process controls to keep the CO/CO2 ratio low, the steam/ hydrogen ratio high, and temperatures outside the critical range can help avoid this attack.3 Metal dusting typically occurs on channels, outlet cones, bypass or shell liners, ferrules, and so on in the waste heat boiler or between the waste heat boiler and 64 Materials Selector for Hazardous Chemicals steam superheater. If this corrosion is experienced, the alloy can be upgraded according to the guidelines (see Table 5.4 under metal dusting) and the severity of the attack.5 Hydrogen embrittlement is common in reformed gas pipelines, which are usually low-alloy steel downstream of the feedwater heaters where the temperature is ⬍400⬚C (⬍752⬚F). This problem can be avoided by selecting conservative materials based on the curves defining safe temperature and hydrogen partial pressure conditions (see Figure 5.1).3 High-Temperature Converter The high-temperature converter, where CO is converted into CO2, is normally made from a low-alloy, 1% Cr, 0.5% Mo steel. The converted gas pipeline is usually type 304 (S30400) stainless steel to avoid corrosion by the acidic condensate. Carbon Dioxide Removal System The materials used in this part of the plant depend on the solvent used to remove CO2. Type 304 or 316 (S30400 or S31600) are commonly used here, but stress-relieved carbon steel is also used for piping and other equipment. Iron in the circulating solution can cause erosion, especially in pumps and bends. In the case of some solvents, such as methyl diethanolamine (MDEA), additives limit corrosion from the carbamates present. In potassium carbonate systems, arsenic and vanadium salts are added to reduce corrosion. A passive layer of magnetite on steel surfaces is maintained by the addition of small amounts of air. If stress corrosion cracking (SCC) of carbon steel is a problem, here duplex stainless steels can provide a solution.3 Waste Heat Recovery System Various grades of ASTM materials such as A213, A312, A335, and A351 provide a useful service life for the boilers and steam superheaters that operate under high heat flux. Control of water quality is also an important factor.3 Ammonia Synthesis If the synthesis gas contains traces of carbon monoxide and carbon dioxide on mixing with the ammonia in the recirculating gas from the synthesis loop, ammonium carbamate will be produced. This material will clog and corrode the downstream equipment. This problem can be avoided only by controlling the carbon oxides level in the fresh makeup gas to less than 5 ppm.3 MS-6: Ammonia and Caustic Soda 65 The ammonia synthesis converter operates at 150 to 200 bar and at about 515⬚C (959⬚F). Nitriding and hydrogen embrittlement can occur in this part of the plant. The pressure shell is usually a multilayer or multiwall carbon-steel vessel. The internal catalyst baskets are usually type 321(S32100) stainless steel. Alloys of chromium (12%–20%), nickel (5%–25%) with molybdenum, vanadium, and tungsten are used for the ammonia converter internals, and ASTM A 213, T22 material of the 21/ 4% Cr, 1% Mo type or their improved versions are used in the boiler. Hydrogen attack can occur in this converter.3 Distillation Columns Distillation columns are often found in recovery systems in chemical processes involving ammonia. Ammonia stripping stills should be fabricated to ASME Code requirements, usually of carbon steel with type 410 (S41000) valve trays. Higheralloy column sections and trays may be required if CO2 contamination occurs to avoid problems of carbamate formation. Heat Exchangers Heat exchangers are used in ammonia production, in ammonia refrigeration units, and in chemical processing. Heaters Tube-and-shell heaters using good-quality steam or an inert heat transfer medium are usually of carbon-steel construction, unless the ammonia or ammonium hydroxide is contaminated. The cooler part of the combustion air preheater at the tail end of the flue gas heat recovery train is likely to corrode as a result of sulfur dioxide condensation from in the flue gas. In this area, cast iron or glass will resist the acid attack. Carbon-steel preheater tubes, with 1.5 to 2 meters of type 304 (S30400) tubes at the cold end of the tube sheet, can also perform well. The high-pressure feedwater heaters are prone to leaks at the tube-to-tube sheet joints. A lining or overlay of the tube sheet with a material such as alloy 600 (N06600) and the use of tube materials such as chromium-molybdenum types reduce this risk considerably. Startup heaters, either electrical or directly fired, are used to heat the synthesis gas feed to the ammonia converter during plant startup. Hydrogen-induced cracks, overheating, flame impingement, thinning at the bends, furnace explosions, and so on are problems encountered in this equipment. Type 321 (S32100) stainless steel is often suitable for startup heater coils and downstream pipeline.3 A number of heaters in ammonia plants have failed and been replaced with 3RE60威 (S31500). These include carbon-steel heat exchangers such as feedwater 66 Materials Selector for Hazardous Chemicals heaters and waste heat boilers that have failed by corrosion and stainless steel exchangers such as shift conversion heaters that failed by SCC.7 Coolers and Condensers Carbon or low-alloy steels may be used, depending on the temperature involved. If the exchanger is water cooled, a stainless steel or other alloy appropriate to the water chemistry should be employed. Copper alloys should not be used in this service. Aluminum-finned steel tubes are used in air-cooled exchangers in ammonium hydroxide service. The compressor interstage coolers are usually made of carbon steel and use water as the cooling medium. Low-velocity areas in the water passage are prone to scaling and microbial corrosion, leading to tube failures. This can be avoided by using improved exchanger design to avoid low-velocity areas and through a proper cooling-water treatment program.3 A number of coolers in ammonia plants have failed and been replaced with duplex 3RE60威 (S31500). These include carbon-steel heat exchangers, such as converter effluent cooler, syngas compressor coolers, and compressor coolers that have failed by corrosion and stainless steel exchangers such as raw gas compressor coolers that failed by SCC.7 Storage Tanks Ammonium hydroxide solutions are usually stored and otherwise handled in carbon-steel tanks or vessels. There are no special requirements, such as heat treatment or impact testing of cylindrical storage vessels, for aqueous ammonia. Because of possible rusting or carbamate corrosion in the vapor phase, type 304 (S30400) may be used where contamination by particulate iron salts is objectionable. Ammonia is stored as a liquid in one of three ways:8,9 • Flat-bottomed steel storage tanks are used for storing very large volumes of ammonia, with typical capacity of 10,000 to 30,000 tonnes (up to 50,000). These tanks are designed for low pressure, so the anhydrous ammonia must be fully refrigerated at atmospheric pressure and at the atmospheric boiling point of –33⬚C. • Semirefrigerated tanks or spheres are used where larger volumes of ammonia must be stored. These are held at some intermediate temperature, such as –12⬚C (10⬚F), between ambient and fully refrigerated (–28⬚F, –33⬚C) conditions. Cylindrical storage vessels (“Bullets”) are also commonly employed. • Ammonia can be pressurized (around 2 MPa, 300 psig) at ambient temperature in spheres or horizontal cylinders up to about 1700 tonnes. Refrigerated Storage Vessels Ammonia storage tanks are usually made from carbon steel after a stress-relief heat treatment to protect against possible SCC (see Chapter 5) and for improved impact properties. Stainless steel, such as type 304 (S30400), is also an acceptable material of construction but is not commonly used. The 31/2% nickel steel has good low-temperature toughness properties and is frequently used as a weld filler metal and sometimes as plates and forgings. MS-6: Ammonia and Caustic Soda 67 There are several construction types of refrigerated storage vessels. The most important are the following: • Single containment: A single-wall insulated tank, normally with a containment bound around it. • Double containment: This type of storage tank has two vertical walls, both of which are designed to contain the stored amount of liquid and withstand the hydrostatic pressure of the liquid. The roof rests on the inner wall. • Full containment: The two walls of this closed storage tank are also designed to contain the stored amount of liquid, but in this case the roof rests on the outer wall. The tank must be constructed in conformity with relevant codes for the construction of pressure vessels or storage tanks based on its design pressure and temperature. The storage tank must be safeguarded against high pressure and in the case of refrigerated liquid ammonia also against a pressure below the minimum design pressure. The ingress of warm ammonia into cold ammonia must be avoided to eliminate risk of excessive evaporation. Tanks must be completely insulated.9 The U.S. standard governing the design, construction, location, installation, and operation of anhydrous ammonia systems, including refrigerated ammonia storage systems, is contained in 29 CFR standard no. 1910.111. The tensile strength of the steel used for construction should be restricted. ANSI standard K-61.110 requires that steels used in fabricating pressure-containing parts of a container shall have a tensile strength no greater than a nominal 480 MPa (70,000 psi). In the United Kingdom, the strength of steels used for spheres and cylindrical vessels is restricted to those having a minimum specified yield (not tensile) strength no greater than 350 N/mm2 (50 ksi). The design, construction, and operation of storage containers is regulated in most countries. Both welding procedures and welders must be qualified to appropriate standards and the tank or vessel thoroughly inspected after fabrication. Tanks and vessels used for containment of anhydrous ammonia must be postweld heat treated as prescribed in the ASME Code. Although some construction codes call for a stress-relief temperature as low as 520⬚C (968⬚F), it is recommended that the minimum temperature should be 593⬚C (1100⬚F) for prevention of SCC. In no event should the PWHT be less than 587⬚C (1050⬚F) tank metal temperature. Impact testing for low-temperature service (e.g. for 31/2% Ni steels) is normally required for cryogenic storage. Type 304 (S30400) is acceptable without PWHT or impact testing. When cryogenic tanks are empty, their inspection can be carried out by a number of techniques. However, emptying these tanks to carry out periodic inspections is difficult and expensive. It is also known that most SCC is initiated during start-up and that even small quantities of residual oxygen can initiate cracking. In some cases it has been found that acoustic emission sensors permanently fixed to such tanks have been useful in detecting defects needing further investigation or remedial action.11 Pressurized Storage Vessels These are also usually carbon steel and designed for up to 2.5 MPa with the pressure of larger cylindrical tanks being limited to about 68 Materials Selector for Hazardous Chemicals 1.6 MPa to avoid the use of steel thicknesses greater than about 30 millimeters. Reflective paint or insulation is added to the outside to avoid solar heating. The shape of the vessel used in this type of storage is largely controlled by its capacity. Cylindrical, usually horizontal, tanks are used for up to about 150 tonnes of ammonia. Spheres are used for 250 to 1500 tonnes.8 Piping For process piping (not gas pipelines) handling anhydrous ammonia, carbon steel is the most common material of construction. As with vessels, stress-relief heat treatment of carbon steels is recommended (or required by regulations) both for protection against possible SCC and for improved impact properties. Types 304 (S30400) or 304L (S30403) are becoming more frequently used as an economical alternative to carbon steel, especially in complex piping systems. The higher material cost can be offset by the elimination of stress-relief heat treatment, impact tests, and painting. Carbon-steel piping is the most common material of construction for ammonium hydroxide and is usually satisfactory if the line is always full. Even if occasionally drained, more resistant alloys are not required unless particulate iron oxides are objectionable. In such cases, aluminum or stainless steel piping may be selected. Type 304 (S30400) or 304L (S40303) is also used where erosion-corrosion is likely. Pumps Anhydrous ammonia or ammonium hydroxide may be pumped in liquid form, while gaseous ammonia is moved by compressors. A pump for liquid anhydrous ammonia is usually of cast steel construction for both impeller and volute. For lowtemperature service, an all–stainless steel pump is preferred. For off-the-shelf items, the standard material is CF8M (J92900), which is usually the most readily available, but low-carbon variants as well as molybdenum-free grades are also suitable. Single-stage centrifugal pumps made from low-temperature steel castings with stainless steel shafts and couplings are used for handling liquid ammonia at –33⬚C.12 Cast steel pumps are routinely used for ammonium hydroxide service. Stainless steel CF8M may be selected to avoid iron contamination. Compressors Cast steel is commonly used, except at temperatures above 300⬚C (570⬚F), where nitriding may occur. At these elevated temperatures, cast austenitic stainless steels (e.g. CF8M) are employed. For steel compressors, the various components may be as follows: Rotor impellers: Type 410 (S41000) stainless steel Stationary parts: Type 410 (S41000) or 304 (S30400) stainless steel MS-6: Ammonia and Caustic Soda 69 Valves As with compressors, cast steel is commonly used except at temperatures above 300⬚C (570⬚F). At elevated temperatures, cast austenitic stainless steels, such as CF8M (J92900), are used. Impact testing of carbon steels for low-temperature service is normally required. Valves for ammonium hydroxide are usually also of cast steel or iron, sometimes with a type 410 (S41000) seat. For throttling valves, stainless steel may be employed. Gaskets, Seals, O-Rings, and Hoses Gaskets are used to seal the metallic or nonmetallic flange faces of pipes. A variety of gasket materials are suitable for ammonia or ammonium hydroxide service, including spiral-wound stainless steel and PTFE, flexible graphite, and compressed asbestos fiber (CAF). For environmental reasons, CAF is restricted or prohibited in many countries but is serviceable where permitted. A gasket design that limits cold flow of PTFE is required at elevated temperatures. This constraint can be mechanical or be achieved by adding resistant fillers to the PTFE, such as glass, silica, or graphite. Another gasketing option is an envelope gasket. This consists of a core of extension-resistant elastomer, sheathed in a thin sheet of fluoroplastic to resist the ammonia. PTFE gaskets used with titanium or zirconium should be made from virgin PTFE, not recycled. There have been cases of fluoride corrosion in gasket areas when recycled PTFE gaskets were used. A newer development provides a soft, easily compressible chemically inert 100% PTFE material with a unique combination of chemical resistance and low-torque requirements. Most grades of Gylon威 PTFE gaskets are suitable for ammonia and ammonium hydroxide at all strengths and temperatures from subzero up to 260⬚C (500⬚F).13 Materials that can be used for O-rings, gasket seals, and hoses in ammonia and ammonium hydroxide service are shown in Table 8.1.14 Ratings given are up to the maximum service temperature unless otherwise noted. Testing is always recommended for seals to be used in specific ammonia environments. It should be noted that some of these elastomers, such as ethylene propylene rubber, may be attacked by entrained oils in compressed gas systems. Natural rubber, hard rubber, isoprene, butyl rubber, and Viton威 A should not be employed. Viton威 Extreme (TBR-S and ETP-S) may be considered at temperatures below 50⬚C (122⬚F). Ammonia should be transferred through articulated arms rather than through hoses wherever possible. Where hoses are used, they should be externally reinforced with type 304 (S30400) stainless steel, while the hose materials themselves may be EPDM, CPR, Buna-N威, or Buna-S威. Fluoropolymers (FEP, PTFE, PFA) and flexible graphite are suitable packing materials. 70 Materials Selector for Hazardous Chemicals Table 8.1 O-Ring Materials Compatible with Ammonia and Ammonium Hydroxide Elastomer Family Anhydrous Ammonia Cold Ammonia Gas Hot Ammonia Gas Concentrated Ammonium Hydroxide 4 4 4 4 1 3 4 4 0 4 3 4 0 0 0 3 4 4 3 2 0 0 0 2 1 4 3 0 4 4 1 1 3 4 0 2 4 4 4 4 1 1 1 2 1 4 1 4 3 4 4 4 1 1 1 4 1 4 1 1 1 4 1 4 Butyl (IR) Chloroprene (CR, neoprene) Chlorosulphonated polyethylene (CSM, Hypalon威, etc.) Epichlorohydrin (CO, ECO) Ethylene-propylene (EPR, EPDM, Nordel威, etc.) Fluoroelastomer (FKM, Viton威 TBR-S, ETP-S) Fluorosilicone (FSI) Natural rubber (NR) Nitrile (NBR, Buna-N威, etc.) Perfluoroelastomer (FFKM, Kalrez威 6375m 1050LF, Chemraz威, etc.) Polyacrylate (ACM) Polysulfide (T) Polyurethane Silicone (SI) Styrene butadiene (SBR) Polytetrafluoroethylene (PTFE, Teflon威, etc.) Key to compatibility: 4 Good, both for static and dynamic seals 3 Fair, usually OK for static seals 2 Sometimes OK for static seals; not OK for dynamic seals 1 Poor 0 No data Bolting Standard bolting appropriate to the strength and temperature requirements is employed (e.g. B7, B8) in both ammonia and ammonium hydroxide service. Transportation Equipment Equipment used for transporting anhydrous ammonia is regulated in most countries. Advice on such regulations can usually be obtained from the manufacturer or MS-6: Ammonia and Caustic Soda 71 supplier of ammonia, often contained in Material Safety Data Sheets (MSDS) or from the local regulatory body. In the United States, the relevant regulations are in the Code of Federal Regulations (CFR) under Title 29 (Labor), Title 33 (Navigation and Navigable Waters), Title 40 (Protection of Environment), Title 46 (Shipping), and Title 49 (Transportation).15 The relevant health and safety rules are incorporated into regulations of the U.S. Department of Labor and Industries.16 With little exception, containers for transportation of anhydrous ammonia are fabricated from carbon or low-alloy steels with some specific requirements as to strength, stress relief, PWHT, and so on to avoid SCC as discussed previously. For shipping containers (including cylinders and DOT-portable containers), cargo tanks, and barges, carbon steel is the most common material of construction. As with storage facilities, type 304 stainless steel (S30400) is also acceptable but not commonly used. The agency regulating transportation in the United States is the Department of Transportation (DOT). The DOT classifies all transportable materials by number and class. Table 8.2 shows this information for ammonia and ammonia solutions. Tanks and containers, approved for the transport of ammonia, must be constructed in accordance with DOT specifications DOT-51, DOT-106A, and DOT-110A. Ammonium hydroxide (NH4OH) is rarely transported in large volumes because it is more economical to transport the compressed gas and make the hydroxide solution by adding water. Small quantities of the reagent grade (28%) is shipped in glass bottles. Polyethylene canisters or metal casks are used for shipping 25% aqueous ammonia.8 However, there will be occasions in chemical processing where ammonium hydroxide needs to be transported. Carbon steel is used, and there are no special requirements, such as heat treatment or impact testing of transportation vessels, for aqueous ammonia. Aluminum and type 304 stainless steel are also suitable. Copperbased alloys and nickel-based alloys lacking a chromium constituent must not be used in this service. Railcar Transport (Tank Cars) For rail transport tank cars, carbon steel is the most common material of construction. Stainless steel (e.g. type 304) is also acceptable but not commonly used. The section of the CFR dealing specifically with ammonia for rail car transport is 49 CFR 173.314. In addition to the federal regulatory requirements, ANSI standard K-61.110 and CGA pamphlet G-2.110 have specific requirements. The design must also be approved by the Association of American Railroads Committee on Tank Cars before the cars are placed in service. Table 8.2 DOT Classifications for Ammonia Concentration DOT No. Anhydrous 10%–35% solution ⬎35%–50% solution ⬎50% solution 1005 2672 2073 1005 125 154 125 125 Classification Nonflammable compressed gas Corrosive liquid Corrosive liquid Corrosive liquid 72 Materials Selector for Hazardous Chemicals Pressure vessels are used for anhydrous ammonia at pressures of about 2.5 MPa. Atmospheric pressure vessels are used for 25% aqueous ammonia with higher pressure vessels (up to 1.6 MPa) for higher concentration ammonia solutions.8 The use of copper, silver, zinc, or their alloys is prohibited. Baffles made from aluminum may be used only if joined to the tank by a process not requiring PWHT of the tank. Tank Trucks For over-the-road transportation in cargo trailers used with motorized vehicles, carbon steel is the conventional material. The use of high-strength lowalloy steels has been restricted or eliminated in some countries. In 1968, a road tanker ruptured in France, killing five people. The investigation into the incident revealed that the cause was stress corrosion cracking, aggravated by fatigue. The material of the tanker was “T-I” steel (A-517) with a tensile strength of 758 to 779 MPa (110– 113 ksi). As a result of this incident, French authorities prohibited the use of T-I steel for storing or carrying ammonia under pressure.17 In addition to federal regulatory requirement, ANSI Standard K-61.110 defines some specific stipulations. Marine Transport For marine transport, the relevant sections of CFR 46 (part 54) apply, specifying applicable pressure/temperature conditions for different shipping modes. These regulations provide limitations and modifications to the requirements of ASME Division 1, Section VIII, for pressure vessels used for marine shipping. Pipelines If the geography permits, transport of large quantities of ammonia by pipeline is more economical over long distances than by river barge or rail. The Mid American Pipeline System (MAPCO) has a total length of 1745 kilometers and extends from Texas to Minnesota, while the Gulf Central Pipeline is a pipeline system with a total length of 3,057 kilometers and connects producers in Texas and Louisiana with terminals in Arkansas, Iowa, Illinois, Indiana, Nebraska, and Missouri. This pipeline was constructed from Grade X42 steel pipe (minimum yield strength 42,000 psi) manufactured to AP1 standard 5LX.18 This grade of pipe was chosen over higher-strength grades (X46 or X52) in recognition of the relationship between ammonia stress corrosion cracking and residual stresses. Ammonia in the pipeline contains a minimum of 0.2% water as an additional precaution. The world’s longest ammonia pipeline has been in operation since 1983 in Russia and connects the producers at Togliatti/Gordlovka with the terminals of Grigoroswski/Odessa some 2424 kilometers away. Ammonia is transported in these pipelines at a temperature of 2⬚C (pressure is between 22 and 100 bar), so it needs to be warmed up at the supplier end and cooled again at the receiver end of the pipe.8 References 1. API 650, “Welded Steel Tanks for Oil Storage” (Washington, DC: API, latest ed.). 2. ASME Section V111, “Pressure Vessels” (New York, NY: ASME International, latest ed.). MS-6: Ammonia and Caustic Soda 73 3. M.P. Sukumaran Nair, “Tackling Corrosion in Ammonia Plants—Selecting the Proper Materials,” Chemical Processing 12, 1 (2001). 4. G.W. Powell, S.A. Mahmoud, eds., Metals Handbook—Failure Analysis and Prevention, vol. 11 (Metals Park, OH: ASM International, 1986), p. 290. 5. S.B. Parks, C.M. Schillmoller, “Improve Alloy Selection for Ammonia Furnaces,” Hydrocarbon Processing (Int. ed.) 76, 10 (1997): pp. 93–98. 6. S.A. Al-Ghafli, H.M. Lari, B. Bousmaha, H.I. Bukhari, “Energy Conservation Measures: Energy Audit, Process Optimization,” IFA Pub. (2003), http://www. fertilizer.org/ifa/publicat/pdf/tech0016.pdf. 7. Anon, “List of References—Heat Exchangers in Ammonia and Urea Plants,” S12311-ENG (Sandviken, Sweden: Sandvik Steel, 1996), 10 pp. 8. M. Appl, Ammonia: Principles and Industrial Practice (Weinheim, Germany: Wiley-VCH, 1999), pp. 209–221. 9. Anon, “Production of Ammonia,” vol. 1 (Brussels, Belgium: EFMA, European Fertilizer Manufacturers’ Association, 2000), 44 pp. 10. Anon, “Safety Requirements for the Storage and Handling of Anhydrous Ammonia,” ANSI K61.1 (New York, NY: ANSI, latest ed.). 11. Anon, “Cryogenic Ammonia Tanks” (Houston, TX: Matrix Inspection and Engineering, Inc., 2000), http://www.matrixie.com/cryogen.pdf. 12. Anon, Dechema Corrosion Handbook, Ammonia and Ammonium Hydroxide Section, CD-ROM (Frankfurt, Germany: Dechema aV, 2001). 13. Anon, “Engineered Gasketing Products,” DPI-8/01 Rev. 0-5M (Palmyra, NY: Garlock Sealing Technologies, 2001), 56 pp. 14. Anon, “O-Ring Compatibilities,: Engineering Fundamentals, Efunda (2002), http://www.efunda.com/DesignStandards/oring. 15. Anon, “Storage and Handling of Anhydrous Ammonia,” Code of Federal Regulations, OSHA (2002). 16. Anon, “Storage and Handling of Anhydrous Ammonia,” Chapter 296-24—Part F-2, U.S. Department of Labor and Industries. 17. A.W. Loginow, E.H. Phelps, “Stress Corrosion Cracking of Steels in Agricultural Ammonia,” Corrosion 18, 8 (1962): p. 229. 18. API 5LX, “High-Test Line Pipe” (Washington, DC: API, out of print since 1982). Section II Caustic Soda 9 Introduction Caustic soda is one of the three most prominent products of the chemical industry, the other two being sulfuric acid and soda ash (sodium carbonate: Na2CO3). By the 1990s, over 13 million tonnes were used annually in the United States alone. Major West European caustic soda capacity in 2003 was estimated to be 11.3 million tonnes/y.1 The annual worldwide production of caustic is of the order of 45 million tonnes.2 As of July 2002, more than 500 companies produced chlor-alkali (a general term to cover the coproduction of chlorine and caustic soda) at over 650 sites worldwide, with a total annual capacity of over 51 million metric tons of chlorine. About half of all plants are located in Asia, but many of these are relatively small.3 A typical chlor-alkali plant is shown in Figure 9.1. This particular plant in Australia produces 85 t/d of caustic using membrane cells. Figure 9.1 Chlor-Alkali Plant in Australia (courtesy of CHEMETICS—a division of Aker Kvaerner Canada Inc.) 77 78 Materials Selector for Hazardous Chemicals Although chemists express the concentration as a percentage, the industrial standard for the sale and use of caustic soda is based on the anhydride (Na2O), wherein 77.5% sodium oxide is equivalent to 100% NaOH. It is the most important commercial, caustic chemical, used in a variety of processes, such as plastics (notably PVC), pulp and paper, soap, glass, aluminum, and so on. It is also used for acid waste neutralization, although soda ash is equally effective and less expensive. In waste management programs, caustic soda is observed to be about 100 times more soluble than lime, which is another, cheaper substitute for such applications. Caustic potash or potassium hydroxide (KOH) is an analogous chemical, sometimes used as a substitute in papermaking processes, but is more expensive and of lesser commercial importance. The term “caustic” is often used to describe both caustic soda and caustic potash. Uses of caustic soda include those listed in Table 9.1.4,5 Table 9.1 Typical Uses of Caustic Soda by Industry Industry Soaps and surfactants Chemical process Oil Petroleum refining Water Food Pulp and paper Pharmaceutical Textile Metal refining Household Uses of Caustic Soda In the hydrolysis of oil and fats In the manufacture of many chemicals, including amyl amines, cresol, ethylene amines, formic acid, glycerine, maleic anhydride, phenol, styrene, vinyl chloride monomer In drilling to control the pH of drilling mud; as a bactericide and calcium remover To remove impurities, such as sulfur, sulfur, and acidic compounds, from the hydrocarbon stream For pH control; regeneration of ion exchange resins; effluent neutralization; descaling of pipe work systems In refining animal and vegetable oils to remove fatty acids; as dry formulations for bottle washing; general cleaning operations; cleaning of brewery equipment; lye peeling of potatoes, fruits and vegetables In the chemical treatment of cellulose fiber from wood to produce cellulose used to make paper; to dissolve lignin from bleached wood pulp as part of the process to produce rayon As a reactant in the manufacture of sodium phenolate used in antiseptics and aspirin As a scouring agent; bleaching scoured cloth; Mercerizing to improve luster and dye absorption To solubilize alumina from bauxite in the ore treatment prior to aluminum production As a precursor of commercial and household cleaners, bleach, and detergents 79 Materials Selector for Hazardous Chemicals References 1. Anon, “Caustic Soda,” European Chemical News 78, June (2003): p. 18. 2. Anon, “Caustic Soda (Sodium Hydroxide)” ChemLink Australasia (1997), http://www.chemlink.com.au/caustic.htm. 3. E. Linak, “CEH Report—Chlorine/Sodium Hydroxide,” SRI Consulting (2002), http://ceh.sric.sri.com/Enframe/Report.html. 4. Anon, “Sodium Hydroxide (Caustic Soda),” Chemistry Store (2002), http://www.chemistrystore.com/Caustic_Soda.htm. 5. Anon, OxyChem Caustic Soda Handbook (Dallas, TX: OxyChem, 2000), 52 pp., http://www.oxychem.com/products/handbooks/caustic.pdf. 10 Properties of Caustic Soda Caustic soda (CAS 1310-73-2) is the common name for sodium hydroxide (NaOH), an alkali also known as sodium hydrate, lye or white caustic. It is a colorless to white, odorless solid or is produced in solutions of various strength in water that are also colorless and odorless. Caustic soda solutions are coproduced with chlorine by a variety of processes (see Chapter 11). The strength and purity of the caustic solution vary, depending on the method of production. More concentrated solutions and solid caustic soda are produced by further evaporative processes. The tolerance range for impurities is dictated by the anticipated service. Different suppliers use different names for grades, depending on the source or use of their product. Commercial solutions can be classified from the purest to least pure product in the following way: • 50% mercury cell grade (also known as rayon or amalgam cell grade) has the lowest salt content of all commercial grades. • 50% purified grade, made from the diaphragm process, has a salt content ⬍0.01% NaCl and can usually be substituted for mercury cell grade. • 50% membrane grade is low in salt (⬍0.01%) and sodium sulfate (⬍0.01%) but also has very low levels of trace metals. • Commercial (or diaphragm cell grade) can be made by diaphragm, mercury, or membrane cells. Contains around 1% NaCl plus trace metals. Solutions at 73% are also available either for subsequent dilution but shipped in concentrated form to save shipping costs or for use in some applications that require this more concentrated solution.1,2 Solid product is typically classified by diminishing purity as reagent grade, mercury cell grade (MCG), rayon grade (low in iron, copper, and manganese), and commercial (i.e. with relatively high levels of impurities). Physical Properties The physical constants of pure sodium hydroxide are shown in Table 10.1.3 Aqueous solutions, of course, have different characteristics. At 50% concentration, the solution freezes at ambient temperatures of less than 12⬚C (54⬚F), and heated 81 82 Materials Selector for Hazardous Chemicals Table 10.1 Physical Constants of Sodium Hydroxide (NaOH) Property Data Molecular weight 40.00 Specific gravity 2.13 Melting point 318.4⬚C (605⬚F) Boiling point 1390⬚C (2534⬚F) Latent heat of fusion 40 cal/g (72 Btu/lb) Heat of formation 101 K/cal/mol Solubility (g/100 cc water) 42 in cold water (0⬚C), 109 at 20⬚C, 347 in hot water (200⬚C) tanks and piping are required. In preparing a 40% concentrated solution, the exotherm generated by the dilution of solid NaOH can elevate the temperature to above the boiling point. Boiling points for various-strength solutions are shown in Table 10.2. The complete range of boiling point and freezing point curves is shown in Figure 10.1.4 It will be observed that the minimum freezing point is at about 25% concentration, which is useful for handling strong acid solutions in plant for neutralization, pH control, and so on. Table 10.2 Boiling Points of Strong NaOH Solutions Weight (%) 10 20 30 40 Boiling Point (⬚C [⬚F]) 103 108 116 128 (218) (226) (241) (262) Chemical Properties Caustic soda is deliquescent, readily absorbing water from the atmosphere to form a film of strong caustic in situ. Solid NaOH reacts vigorously and exothermically when added to water, as in making caustic solutions for various purposes. The concentration of caustic soda solutions is related to its pH in Table 10.3.3 Because of the difficulty of obtaining accurate pH readings at values above 12, pH is not a valid method to determine concentration of caustic soda solutions. Caustic soda rapidly absorbs carbon dioxide from the air, forming sodium carbonate. It should be kept away from heat, sparks, or flames and not stored or mixed with incompatible materials. Caustic soda is incompatible with the following ma- MS-6: Ammonia and Caustic Soda Figure 10.1 83 Range of Boiling and Freezing Temperatures of NaOH terials: water; acids; flammable liquids; organic halogens; metals such as aluminum, tin, and zinc; and nitromethane. It is corrosive to some metals and can generate hydrogen gas. Contact with water may generate sufficient heat to ignite combustible materials. Carbon monoxide gas can form on contact with food and beverage products in enclosed spaces and can cause death.5 Table 10.3 Hydrogen Ion Concentration of Various-Strength Caustic Soda Solutions at 25⬚C (77⬚F) % NaOH 7.40 3.83 1.96 0.39 0.20 0.04 Moles/L pH 2.0 1.0 0.5 0.1 0.05 0.01 14.0 13.8 13.6 12.9 12.6 12.0 84 Materials Selector for Hazardous Chemicals Safety and Health Considerations The word “caustic” is defined as “destructive or corrosive to living tissue; an agent which burns or destroys living tissue.”6 Given that description and the common name for sodium hydroxide, it is hardly surprising that it is highly toxic by either ingestion or inhalation and is a strong irritant to the eyes, skin, and mucous membranes; that is, it is “corrosive” in the physiological sense. Beside potential burns to the eyes or skin, fire may produce irritating or poisonous gases that are harmful if inhaled. Various regulatory bodies set exposure limits intended to protect humans who might come into contact with caustic soda. Current limits are as follows:7,8 OSHA Standards: permissible exposure limit (PEL): TWA: 2-mg/m3 ceiling ACGIH threshold limit value (TLV): 2-mg/m3 ceiling NIOSH: recommended exposure limit (REL): 2-mg/m3 ceiling Immediately Dangerous to Life or Health (IDLH): 10 mg/m3 Recommended Protective Equipment Exhaust ventilation should be provided to maintain airborne concentrations below recommended exposure guidelines. When exposure levels could exceed 2 mg/m3, a NIOSH-approved air-purifying, full-face respirator with high-efficiency particulate filters is recommended. When exposure levels could exceed 10 mg/m3, a selfcontained breathing apparatus with a full face piece is recommended. Protective clothing impervious to caustic such as neoprene or polyvinyl chloride (PVC) should be used when handling caustic. Precautions should be taken to ensure that all potentially affected body parts are covered, such as taping sleeves and pant legs to gloves and boots, respectively, and buttoning clothing to the neck. Selection of specific items such as gloves, coats, pants, boots, aprons, or full-body suits will depend on operations to be performed. Avoid leather and wool. A safety shower should be located in the immediate work area. Contact lenses should not be worn; they could contribute to severe eye damage. Wear close-fitting chemical splash goggles as a minimum. Where there is a possibility of splashes to the face, a full-length transparent face shield should be worn.9 Fire and Explosion Sodium hydroxide itself will not burn, but fires may occur. If the solution temperature is raised by fire, caustic attacks steel with liberation of hydrogen gas that will burn. Also, other combustibles, such as wood, paper, or oil, may ignite from local overheating. If caustic soda is exposed to a fire, sodium oxide fumes may be generated. In the event of fire or explosion, the following precautions should be taken: MS-6: Ammonia and Caustic Soda 85 • • • • Keep unauthorized people away. Stay upwind and avoid low areas. Isolate the hazardous area and deny further entry. Wear self-contained breathing apparatus (SCBA) and full protective clothing and gear. • If possible to do so safely, remove container from the fire area. If not, cool the fireexposed containers with water until flames are fully extinguished. Avoid contacting caustic soda with water if at all possible. Small fires can be extinguished using dry chemicals, carbon dioxide (CO2), water spray, or foam. Larger fires should be controlled with water spray or foam. First Aid Victims should be moved to a safe area with fresh air and an emergency team summoned. Contaminated clothing and shoes must be removed and isolated. In case of fluid contact, flush skin and/or eyes with fresh, running water for at least 15 minutes. In case of inhalation, remove to fresh air and supply oxygen and/or artificial respiration if necessary. In case of ingestion, do not induce vomiting, give large quantities of water if victim is conscious, and seek immediate medical help. Disposal, Spill, or Leak Procedures Runoff from fire control or water washing may cause pollution, although the sodium ion itself is nonpolluting. Caustic spills to water are toxic to animal life, so contain and neutralize with dilute acetic or hydrochloric acid if possible. Caustic Dilution Caustic is often shipped in solid form or in more concentrated solutions than is required by the end user. This is done to reduce shipping costs and means that the end user must be able to dissolve or dilute caustic safely to produce the required concentration. The precautions necessary to carry out this dilution process include the following: • Always add caustic soda solid or solution to water with constant agitation. Never add water to caustic soda solutions. • The water used should be lukewarm at 80⬚F to 100⬚F (27⬚C–38⬚C). Never start with hot or cold water. Caustic soda, solid or solution, has a high heat of solution so that the addition of caustic soda to water or more dilute solution will cause a rise in temperature. If 86 Materials Selector for Hazardous Chemicals caustic soda becomes concentrated in one area, is added too rapidly, or is added to hot or cold liquid, the temperature increase can be very rapid. This can result in the formation of dangerous mists or boiling and spattering, which may cause an immediate violent eruption. References 1. Anon, “Caustic Solution Forms” (Midland, MI: Dow Chemical, 2003), 2 pp. 2. Anon, “Caustic Soda” (Cleveland, TN: Olin Chlor Alkali Products, undated), 48 pp. 3. Anon, “OxyChem Caustic Soda Handbook” (Dallas, TX: OxyChem, 2000), 52 pp., http://www.oxychem.com/products/handbooks/caustic.pdf. 4. Anon, “OxyChem Caustic Soda Handbook” (Dallas, TX: OxyChem, 2000), p. 29, http://www.oxychem.com/products/handbooks/caustic.pdf. 5. Anon, “MSDS NAOH50” (Newark, CA: Jones-Hamilton Co., 2002), 8 pp. 6. T.C. Collocott, A.B. Dobson, eds., Chambers Science and Technology Dictionary (Edinburgh, UK: W&R Chambers Ltd, 1984), p. 196. 7. R.J. Lewis, ed., Sax’s Dangerous Properties of Industrial Materials, 10th ed. (New York, NY: John Wiley & Sons, 2000), p. 3253. 8. Anon, “NIOSH Pocket Guide to Chemical Hazards,” NTIS no. PB 91-151-183/ A07 (Cincinnati, OH: NIOSH, 1990), 250 pp. 9. Anon, “MSDS NAOH50” (Newark, CA: Jones-Hamilton Co., 2002), 8 pp. 11 Production of Caustic Soda Sodium hydroxide can be made in a reversible reaction by treating sodium carbonate with quicklime. The reaction cannot go to completion, so it is difficult to convert more than about 90% of the carbonate into hydroxide. The majority of caustic soda is made commercially, however, as a by-product of chlorine production by the electrolysis of sodium chloride solutions. One ton of salt produces 0.58 tons of chlorine and 0.63 tons of caustic soda.1 There are three major processes used in the manufacture of chlorine and caustic soda. The share of production among these processes, on a worldwide basis, is approximately as follows: • • • • Diaphragm cell: 75% Mercury cell: 19% Membrane cell: 3% Other technologies: 3% This distribution is not uniform throughout the world, however, since the dominant technology varies in different regions as follows: • Western Europe, predominance of mercury cell process (June 2000): 55% • United States, predominance of diaphragm cell process: 75% • Japan, predominance of membrane cell process: ⬎90% The remaining chlorine production capacity in western Europe consists of diaphragm cell process 22%, membrane cell process 20%, and other processes 3%.2 In 2003, the technology being employed throughout Europe was distributed as follows:1 • Diaphragm technology: 25% • Mercury cells: 64% • Membrane process: 11% 87 88 Materials Selector for Hazardous Chemicals The supply of sodium hydroxide (NaOH) is diminishing because of a decreasing demand for chlorine. At the same time, production of chlorine and caustic soda by membrane cells is increasing at the expense of mercury cell technology throughout the world. There are a number of factors driving the decision about which technology to use to produce chlorine and caustic, including costs of electricity, environmental pressures, and so on. The commercial products vary in purity, the kind and amount of impurities determined largely by the specific process employed in manufacture. For example, diaphragm cell caustic can contain up to 5000 ppm chloride ion compared with 20– 30 ppm Cl– in the product from mercury cells. When high-purity grades are required, it is necessary to take precautionary measures to maintain product quality. The very highest purity caustic, of less than 2 ppm iron content, is produced by an advanced membrane gap cell (MGP) technology in which high-molecular-weight impurities are separated from the caustic. Iron pickup is avoided by using materials other than carbon steel, the conventional material of construction, in manufacture, storage, and transport. Other materials utilized for this purpose include nickel-based alloys, stainless steels, and silver (in special cases). Storage and transport compartments for liquid products may be coated with epoxy coatings or with electroless nickel plating (ENP). Other developments in membrane cell technology being investigated include the use of oxygen consumption instead of hydrogen evolution as the cathodic reaction. Related developments include changes to the cathode materials, proprietary coatings, and so on; modifications to the anode structure; and the use of zero gap cell technology. This research is aimed at increasing energy efficiency and product purity.3 Production Processes Of the three conventional processes, the largest production is derived from diaphragm cells. Materials of construction for the production and handling of caustic soda are discussed in subsequent chapters. Since brine preparation, construction of chlorine cells, and handling of chlorine is described in detail in MTI MS-3,4 those aspects of caustic soda will not be addressed in this monograph. Diaphragm Cell Process In the diaphragm cell, a naturally occurring brine or a salt solution, dissolved in rubber- or brick-lined tanks, is subjected to electrolysis by direct current. In the former case, the brine must be pretreated to remove certain impurities (e.g. calcium, iron, manganese, and sulfate species). A caustic solution (10%–15%) is produced at an iron cathode. In the anodic reaction on the other side of the asbestos diaphragm, chlorine and hydrogen are evolved from the solution comprising about 15% each of caustic and sodium chloride. MS-6: Ammonia and Caustic Soda 89 Since the crude caustic product is heavily contaminated by iron, sodium chlorate, sodium chloride, and dissolved chlorine, further treatment is required. Chemical treatments include additions of liquid or other chemicals, evaporation, and salt separation. Asbestos-based diaphragm cells are under pressure to be phased out in western Europe.5 Mercury Cell Process The mercury cell uses a rubber-lined cell in which a solution of approximately 25.5% sodium chloride concentration is electrolyzed, diminishing in concentration to about 21% as the reaction progresses. The anode may be graphite or titanium and releases chlorine and hydrogen as products. The cathode is an inclined steel plate over which runs a molten mercurysodium amalgam that is bled off to a separate compartment where hydrolysis with demineralized water produces a 50% caustic of exceptional purity. The mercury cell process is being phased out because of environmental problems occasioned by release of mercury. European producers have committed to convert mercury plants to membrane technology by 2020.5 Membrane Cell Process The membrane cell is analogous to the diaphragm cell except that the feed brine is more highly purified and the perfluorosulfone membrane has lower permeability than a diaphragm. Consequently, a high-quality concentrated sodium hydroxide is produced. The caustic product from membrane cells is around 30% NaOH. A typical membrane cell is shown schematically in Figure 11.1.6 Figure 11.1 Schematic View of Membrane Cell Showing Inputs and Outputs 90 Materials Selector for Hazardous Chemicals Concentrated Solutions and Solid Caustic Soda The weak caustic solutions from the membrane or diaphragm processes are first purified to remove chlorates, chlorides, iron, chlorine, and so on and then concentrated to produce a salable, usable product. Once the caustic soda leaves whichever type of chlorine production cell that was used, it must be filtered and purified to a lesser or greater extent before being ready for use or sale. Mercury cell caustic is generally the most pure and does not usually need to be concentrated further unless 73% or solid caustic is being produced. The diaphragm cell caustic is generally the most impure and the weakest. The three processes and the treatment of the caustic soda product are shown schematically in Figure 11.2.7 After purification, multistage evaporators are used to increase the concentration by evaporation (see Figure 11.3). In this figure, showing a triple-stage evaporator, FE is the forced evaporator and CR is the crystallizer.8 Since diaphragm cell caustic soda is the most widely produced, the handling of this product will be described further. The product from the other processes is treated similarly with the differences shown in Figure 11.2. Once the caustic soda leaves the cell, sodium chlorate must be removed to prevent corrosion at elevated temperatures. The chlorate is usually extracted with ammonia, but other methods, often proprietary, are also used. Sodium chloride concentrates and crystallizes during the caustic concentration and must be removed by settling and filtering. The purified caustic is fed to the evaporators, where the solution is concentrated up to nominally 50% or 73%, depending on the process and the use for which the caustic is required. If solid product is required, all the water is evaporated from the solution and the liquid, anhydrous NaOH, is allowed to cool and solidify. Flake solid is made by passing molten caustic over cooled flaking rolls. The flakes can then be milled to the required particle size. Caustic soda beads are produced from molten caustic in a prilling tower that produces beads of uniform shape and size.7 The solids are deliquescent and so require continual protection against exposure to atmospheric humidity. Materials that are used in this concentration and purification process are discussed in Chapter 18. Impurities Contaminants in caustic may arise from production processes, storage, and handling or from chemical processes employing NaOH as a constituent or additive (e.g. for neutralization or emulsification). In the production of caustic by the mercury cell process, the feedstock brine may introduce calcium, iron, manganese, sulfates, and hypochlorites as well as traces of mercury. In diaphragm processes, contaminants make up such species as unreacted sodium chloride, sodium chlorate, chlorine, and sodium sulfate. In other process applications, even a change in chemical grade may lead to unexpected corrosion, and corrosion/materials engineers need good communications channels with sup- MS-6: Ammonia and Caustic Soda Figure 11.2 91 Block Diagram of the Production of Caustic Soda from the Various Types of Electrolytic Cells pliers, designers, and operating personnel. The effect of these impurities and mixtures is discussed in Chapter 15. References 1. Anon, “Keywords—Chlorine,” European Salt Producers’ Association (2003), http://www.eu-salt.com/manufact/chlorine.htm. 92 Materials Selector for Hazardous Chemicals Figure 11.3 Flow Diagram of Triple-Effect Caustic Soda Evaporator 2. Anon, “Reference Document on Best Available Techniques in the Chlor-Alkali Manufacturing Industry,” European Commission, Brussels (2001), http:// www.envir.ee/ippc/docs/chlor-alkali.doc. 3. J. Chlistunoff, “Advanced Chlor-Alkali Technology,” IMF Program Review Meeting, Golden, CO (2003), http://www.oit.doe.gov/imf/pdfs/revpres_17_ chlistunoff.pdf. 4. C.P. Dillon, W.I. Pollock, eds., Materials Selector for Hazardous Chemicals: Hydrochloric Acid, Hydrogen Chloride and Chlorine, vol. MS-3 (St. Louis, MO: MTI, 1995), 200 pp. 5. Anon, “Caustic Soda,” European Chemical News 78, June (2003): p. 18. 6. Anon, “Chloralkali Technology” (Vancouver, BC: Chemetics—A Division of Aker Kvaerner, 2003), p. 5. 7. Anon, “OxyChem Caustic Soda Handbook” (Dallas, TX: OxyChem, 2000), p. 4. 8. C.M. Schillmoller, “Alloy Selection for Caustic Soda Service,” NiDI technical series no. 10019 (Toronto, ON, Canada: NiDI, March 1988), p. 8. 12 Corrosion by Caustic Soda Corrosion is the deterioration of a material by reaction with its environment. For metals and alloys, this is mostly an electrochemical process involving anodic and cathodic reactions. Corrosion rates in aggressive chemicals usually decrease as the pH increases. In alkaline solutions, the hydrogen ion is present in very low concentrations. However, many metals pass through a minimum corrosion rate at some pH, usually basic, and then suffer increased corrosion as pH continues to rise. Aluminum can liberate hydrogen ions from basic solutions. Since hydrogen ions are in short supply, it is likely that the cathodic reaction in alkaline media involves absorbed water molecules, such as H2O Ⳮ eⳮ r OHⳮ Ⳮ H (1) while the anodic reaction remains the same as in acidic corrosion: M r MⳭ Ⳮ eⳮ (2) The metal ion is removed from solution by forming a basic salt, such as a ferroate, aluminate, or zincate. Quite often, corrosion by alkalis leads to pitting and other localized attack because they tend to form cathodic films, and attack is concentrated at susceptible anodic areas. Carbon and low-alloy steels, austenitic stainless steels, and some nickel alloys may suffer either stress-corrosion cracking or general corrosion in hot, concentrated caustic. Passivity Stainless steels and some other iron-chromium and nickel-chromium alloys, as well as titanium, zirconium, and aluminum, achieve “surface passivity” by developing 93 94 Materials Selector for Hazardous Chemicals a tenacious surface oxide layer that is relatively inert. Interruptions to this film by inclusions, embedded metal particles, or mechanical breaks provide unprotected sites for corrosion to occur. In the case of austenitic stainless steels, inclusions and other foreign materials can be removed from the surface by immersion in 20% nitric acid at about 75⬚C (167⬚F). The purpose of this treatment is to clean the metal surface and remove embedded particles before allowing the oxide film to reform in a more continuous, thicker, and more tenacious form than might occur naturally. A similar process involving the use of caustic solutions is applied to aluminum alloys. The protective surface oxide reforms spontaneously on reexposure to air. Sodium hydroxide (NaOH) is a strong alkali and therefore corrosive to amphoteric metals (i.e. metals able to form salts with or be corroded by both bases and acids), such as aluminum, lead, and tin, even in dilute solutions at room temperature. These are directly attacked, liberating hydrogen just as one observes with base metals in a mineral acid and forming salts (i.e. sodium aluminate, plumbate, or stannate) by such reactions as shown here:1 Al Ⳮ NaOH Ⳮ H2O r NaAlO2 Ⳮ 11/2 H2 (3) A similar type of direct corrosion occurs with, for example, ferrous metals (forming sodium ferroate) but only at higher concentrations and temperatures. Behavior of metals in caustic is governed by their position in the EMF series. Those anodic to hydrogen (e.g. ferrous metals) are attacked by caustic with evolution of hydrogen under certain conditions. Those cathodic to hydrogen (e.g. copper and alloys) are generally resistant in the absence of oxidizing contaminants. As with any corrosive environment, contaminants in caustic or admixtures with other chemicals can dramatically change the reactivity with specific materials of construction. Forms of Corrosion Of the many forms of corrosion that have been identified, general corrosion predominates with nonresistant metals and alloys, while the resistant alloys are more likely to suffer some form of localized attack. The most common forms of localized attack are intergranular corrosion of stainless steels and crevice corrosion. The forms of corrosion described in this chapter are encountered with one material or another under certain conditions of temperature, concentration, or contamination. The corrosion behavior of specific materials is discussed in detail further in Chapters 13 to 15. General Corrosion General or uniform corrosion is the common form of metal loss in most corrodents in the absence of passivating films. In this form of corrosion, the metal is removed MS-6: Ammonia and Caustic Soda 95 uniformly over the entire exposed surface. Ferrous alloys can suffer from this form of corrosion, as can nickel, copper, and titanium in the presence of oxidizing species. Localized Corrosion Pitting corrosion (in which the attack takes the form of small but deep cavities) or other localized attack (e.g. concentration cell corrosion, crevice attack, and so on) may be encountered under some circumstances, especially with alloys that depend for their corrosion resistance on a passive film. This is due to the ability of caustic to produce cathodic oxide films, concentrating the attack at small anodic areas where the film is defective. Galvanic Corrosion This does not seem to be a practical problem in caustic soda. Zinc is anodic to copper in this environment, but the usual strong cathode/anode relationship between carbon and iron is not encountered. Erosion-Corrosion This is a form of corrosion resulting from the loss of protective surface oxide from excessive velocity or turbulence of contacting fluids. This problem is controlled by alloy selection or by limiting the velocity of fluids in pipes to less than about 2 mps (6 fps). Centrifugal pumps often exhibit this type of corrosion. Intergranular Attack Under some circumstances, brasses or bronzes made with zinc may suffer intergranular attack (IGA). In hot, concentrated caustic soda solutions, carbon steels can suffer IGA. Austenitic and ferritic stainless steels can suffer IGA due to chromium depletion at grain boundaries caused by heating in a critical temperature range. Dealloying Although gray cast irons are not prone to graphitic corrosion, high-zinc brasses will suffer a selective dissolution of the zinc-rich phase. It has recently been shown that high-performance nickel alloys can be subject to dealloying attack in hot, caustic soda solutions. Field failures from this mechanism have also been reported.2 96 Materials Selector for Hazardous Chemicals Liquid Metal Embrittlement Liquid metal embrittlement (LME) is a form of attack in which metals that are molten at operating temperatures penetrate the grain boundaries of a solid metal or alloy and cause extensive mechanical damage. It is also known as liquid metal cracking. Mercury deriving from mercury cell production can cause LME of alloys, such as alloy 400. Titanium and zirconium and their alloys, copper and its alloys, aluminum and its alloys, and alloy 200 at elevated temperatures are also known to be at risk from this form of attack.3 Stress Corrosion Cracking Stress corrosion cracking (SCC) is an environmentally assisted form of attack that results from interaction between the environment and a specific alloy system under tensile stress. It is a temperature-sensitive phenomenon. Classical examples of SCC are copper alloys in ammonia, steel in caustic solutions, and stainless steels in chloride environments. SCC is the most common corrosion phenomenon associated with caustic. In steels, it is often called “caustic embrittlement,” a misnomer in that ductility of the metal is unimpaired in the matrix. SCC is also encountered with stainless steels under some conditions (see the later discussion). Nickel-base alloys are very resistant but not totally immune. In the laboratory, SCC has been induced in 70 to 30 brasses but only at artificially induced potentials; it is not a problem in actual service utilizing high-strength copper alloys. The first identified cases of SCC were in riveted carbon-steel boilers that failed by caustic cracking. The caustic soda used to treat the boiler feedwater became concentrated in crevices at the riveted joints, which were highly stressed.4 In the mid- to late 1800s, as steam power became more widespread, boiler explosions were common, causing a great number of casualties and major damage. Caustic cracking occurs within a defined range of temperature and caustic concentrations. For example, at about 10% NaOH, caustic SCC is possible at temperatures ⬎80⬚C (176⬚F), while at 20%, it may occur at temperatures ⬎60⬚C (140⬚F). High-Temperature Corrosion Very hot caustic tends to strip otherwise protective oxide films from alloy systems. At 800⬚C to 815⬚C (1470⬚F–1500⬚F), molten caustic attacks even high-nickel alloys by selective dissolution of iron, chromium, and molybdenum, leaving a porous surface. Heat transfer through a metal to the caustic solution also accelerates corrosion in many cases. The mechanism is by increasing the caustic concentration locally and by erosion caused by the formation of gas bubbles. MS-6: Ammonia and Caustic Soda 97 References 1. H.H. Uhlig, R.W. Revie, Corrosion and Corrosion Control, 3rd ed. (New York, NY: John Wiley & Sons, 1985), p. 345. 2. G. Chambers, “Caustic Dealloying Corrosion of High Performance Nickel Alloys,” Stainless Steel World, October 2003, pp. 57–59. 3. J.R. Davis, ed., Corrosion—Understanding the Basics (Materials Park, OH: ASM International, 2000), p. 191. 4. H.H. Uhlig, Corrosion and Corrosion Control (New York, NY: John Wiley & Sons, 1971), p. 129. 13 Corrosion of Metals and Alloys The corrosion characteristics of the metals and alloys that might be considered for service in solutions of nominally pure sodium hydroxide, at various concentrations and temperatures, are discussed in this chapter. The effect of contaminants or mixtures is discussed in Chapter 15. Materials of construction for specific plant items and types of equipment are described in Chapter 18. Aluminum and Its Alloys Aluminum is rapidly attacked by even dilute solutions of caustic soda at all temperatures. The aluminum ion (AlⳭⳭⳭ) is readily complexed by hydroxyl ions (OHⳮ). Dilute solutions (1 N) can be inhibited by saturating with potassium dichromate. Aluminum should not normally be considered for service above about pH 8.5.1 The corrosion of aluminum in caustic is controlled by the competing processes of film growth and dissolution. The film formed consists of an inner compact layer and an outer crystalline one. At pH around 9 or less, the corrosion rate is low, but at higher pH, cavities form in the outer layer, permitting access of the fluid to the surface and increasing corrosion rate. At pH 12, these cavities are more prominent, and severe localized corrosion occurs. The corrosive effect of increasing solution pH for aluminum at different temperatures is clearly seen in Figure 13.1.2 The addition of 1,000 ppm chloride ions decreased the corrosion rate at 60C (140F) but had no appreciable effect at 30C (86F). Impurities present in NaOH can have a strong effect on the corrosion of aluminum. Impurities such as Fe2Ⳮ can be reduced on the metal surface, forming preferential sites for hydrogen evolution and greatly increasing corrosion current. Aluminates in solution contribute to a slight decrease in corrosion current.3 99 100 Materials Selector for Hazardous Chemicals Figure 13.1 Effect of pH on the Corrosion Rate of Aluminum in NaOH at 30C (left) and 60C (right) Iron and Steel Ferrous alloys are very commonly used in caustic soda service, provided iron contamination is not objectionable and provided certain restrictions are imposed on service conditions to avoid stress corrosion cracking (SCC). At ambient temperatures, iron and steel are protected by a passive layer of magnetite that is formed by the following reaction: 3Fe Ⳮ 4 OHⳮ r Fe3O4 Ⳮ 4Hⳮ Ⳮ 4eⳮ (1) Magnetite is the least soluble iron oxide in alkaline solutions, so corrosion is largely prevented under these conditions.4 At elevated temperature, magnetite has been found to be either protective or nonprotective, depending on the growth conditions. A protective film grows at the metal/oxide interface with “excess” iron ions forming soluble ferroates, while the nonprotective magnetite film becomes highly stressed as it grows at the oxide/solution interface. The breakdown of the protective film also occurs at more elevated temperatures and under turbulent or erosive conditions.5 Cast Irons Gray cast iron is resistant to caustic but is usually not used because of safety problems associated with its brittle nature. At one time, caustic soda solutions were concentrated in cast iron evaporators. Hot alkalis at 30% concentration attack unalloyed irons. Temperature should be 80C (175F) in concentrations up to 70% if the corrosion rate is not to exceed MS-6: Ammonia and Caustic Soda 101 0.25 mm/y (10 mpy). The corrosion rate of ductile cast iron (DI) is similar to that of gray cast iron, but DI can be susceptible to cracking in highly alkaline solutions, while gray cast iron is not.6 Ductile cast iron is, however, sometimes used for specific items, such as valves or pumps. The addition of nickel greatly reduces the corrosion rate of cast iron in boiling 50% to 65% caustic, as shown in Table 13.1.7 This was an 81-day test under a vacuum of 660 mm (26 in.) mercury The austenitic nickel cast irons of greater than about 15% nickel content, such as NiResist威 types 1 (F41000) and 2 (F41002) and the ductile NiResist type D2 (F3000), are much more resistant than unalloyed cast iron in caustic solutions up to about 70%. Corrosion rate should be 0.25 mm/y (10 mpy) in solutions up to 70% NaOH at temperatures approaching boiling.6 The resistance is roughly proportional to the nickel content unless sulfur or sulfur compounds are present. The nickel-containing cast irons may be susceptible to SCC, especially in the presence of high chlorides, so it is considered a reasonable precaution to stress relieve these alloys before use in hot caustic soda solutions.7 The corrosion rate of NiResist威 cast iron is compared with that of gray cast iron in Figure 13.2.8 Detailed corrosion data for NiResist威 are compared with those of unalloyed cast iron in Table 13.2.9 The resistance of unalloyed and alloy cast irons in molten caustic soda is shown in Table 13.3.10 These data show that nickel has superior resistance to all these cast irons, including the highly alloyed ones. High-silicon cast irons have good resistance to relatively dilute caustic soda solutions at moderate temperatures. This type of iron, such as the 14% silicon cast iron (F47003), is not resistant at higher strengths of caustic at elevated temperature because NaOH reacts with the siliceous film from which it derives its acid resistance. High-chromium cast irons are also not resistant to strong alkaline solutions. Carbon and Low-Alloy Steels Some early data on the corrosion of steel in ambient temperature is shown in Table 13.4. These data show that corrosion rate decreases with an increase of caustic concentration at room temperature.11 At ambient temperature, steels are only slightly attacked by caustic soda with solution strength having little effect on rate. Unalloyed and low-alloy steel corrode at 0.005 mm/y (0.2 mpy) in up to 20% NaOH at ambient temperature. In stronger solutions, 20% to 50%, the corrosion rate will be 0.01 mm/y (0.4 mpy), and the steel is still usually protected by the presence of a passive layer of magnetite.4 At higher strengths and temperatures, this oxide layer no longer provides effective protection, and corrosion rates increase. The stated limits for the use of carbon and low-alloy steels in caustic soda solution at temperatures above ambient vary widely. Typical suggested limits on the basis of metal loss by corrosion are 50% concentration at temperatures up to 85C to 90C (185F–194F)12 and 50% at up to 60C (140F).13 One source says that the corrosion rate in 50% will be 1 mpy (0.025 mm/y) at 40C (104F), 5 mpy (0.13 mm/y) at 60C (140F), and 8 mpy (0.20 mm/y) at 55C to 75C (131F–167F).14 Yet another 102 Materials Selector for Hazardous Chemicals Table 13.1 Effect of Nickel Additions on Corrosion of Cast Irons in Boiling 50% to 65% NaOH Nickel (%) 0 3.5 5 15 20 20 (Ⳮ2% Cr) 30 Corrosion Rate (mm/y [mpy]) 1.9 (73) 2.3 (91) 2.2 (86) 1.2 (47) 1.24 (49) 0.8 (30) 0.08 (3.3) 0.15 (6) 0.01 (0.4) Figure 13.2 Corrosion Rates of Gray Cast Iron Compared with NiResist威 in Caustic Soda MS-6: Ammonia and Caustic Soda 103 Table 13.2 Corrosion Data for NiResist威 and Cast Iron in Caustic Soda Medium 50% caustic soda plus suspended salt 50% caustic soda 50% caustic soda 50% caustic soda Anhydrous sodium hydroxide 75% caustic soda NaOH and KOH each 90% Caustic soda and dissolved silicates Location Salt tank Distributor box to settler Evaporator High-concentration evaporator Flaker pan Storage tank after vacuum evaporator Flaker pan Drop kettle in metal cleaner manufacture Average Corrosion Rate (mpy [mm/y]) Temperature F (C) Cast Iron NiResist威 180 (82) 6.3 (0.16) 0.01 (0.01) Hot 20 (0.51) 4 (0.10) Hot Hot 30 (0.76) 40 (1.02) 6 (0.91) 20 (0.51) 700 (371) 510 (12.95) 13 (0.33) 275 (135) 70 (1.78) 4 (0.10) 700 (371) 500 (12.7) 13 (0.33) 120 (49) 30 (0.76) 5 (0.13) Table 13.3 Corrosion of Cast Irons by Molten NaOH at 510C (950F) Material Gray iron Ductile iron White iron 3% nickel-iron Austenitic, type 1 Austenitic, type 2 Ductile austenitic, type 2 Austenitic, type 3 Austenitic, type 4 Wrought nickel Corrosion Rate (mm/y [mpy]) Pit Depth (mm [mils]) 2.5–3.4 (97–135) 5.3 (207) 3.8 (151) 1.8 (71) 15.9 (628) 24.2 (954) 11.8 (466) 2.2 (87) 13.6 (534) 0.23 (9) 0.13 (5) — 0.5 (20) — 1.5 (60) 1.8 (70) 1.5 (60) 0 1.0 (40) — 104 Materials Selector for Hazardous Chemicals Table 13.4 Corrosion of Steel by Sodium Hydroxide Solutions NaOH (g/L) 0 0.001 0.1 1.0 10 100 540 Corrosion Rate (mpy [mm/y]) 2 (0.051) 2 (0.051) 2 (0.051) 0.7 (0.018) 0 0.1 (0.003) 0 source says that the corrosion rate in 50% will be 2.5 mpy (0.06 mm/y) or less at 70F to 100F (21C–38C).13 Some of the discrepancy between various stated safe limits is caused by other factors, such as purity of the caustic soda, velocity, oxygen, concentration effects leading to caustic SCC, and so on. In other strengths of caustic, there is less discrepancy, and typically carbon steel is said to be satisfactory up to 70% at up to 80C (176F)15 and in 75% at up to 100C (212F), assuming that iron contamination is acceptable.16 These limits of operating conditions are based on reasonable rates of metal loss and assume that iron contamination of the solution is acceptable. If this is not the case, then a more resistant material must be employed or the vessel must be lined. Storage tanks are often lined with neoprene, phenolic-epoxy, or other resistant coating or lining. In the absence of concerns about iron contamination, bare steel tanks are used effectively to store caustic solutions up to about 50% concentration and up to about 65C (150F).6 If steel is under tensile stress, as from welding or cold work (e.g. field flaring of pipe flanges) and is exposed to caustic soda, caustic SCC is possible. Under these conditions, a safe operating limit might be up to 50% caustic at up to 65C, although cracking has occurred at temperatures as low as 48C (118F).17 A survey of field experience was used to produce a curve that indicated temperatures and caustic strengths above which cracking was possible. These data were thought to be conservative but were based on many years of practical experience.18 Another curve depicting the parameters of caustic concentration and service temperature above which caustic cracking may be a problem was produced from short-term (up to 62 days) laboratory tests (see Figure 13.3).19 This curve is also thought to be conservative but is widely used and reproduced in various forms. Failures that occasionally occur in the safe zone are likely to be associated with other factors, such as localized overheating or contaminants present in the caustic. Figure 13.4 shows the suggested regions, as defined by temperature and concentration, in which postfabrication thermal stress relief (or an alternative alloy) is recommended to avoid SCC in steels.20 An iron alloy with 3% nickel was found to be immune to caustic cracking, and the addition of 0.5% molybdenum had little effect on that SCC resistance. However, segregation of phosphorus at grain boundaries significantly reduced resistance. Sim- MS-6: Ammonia and Caustic Soda 105 Figure 13.3 Temperature and Concentration of Caustic Soda that Can Cause SCC of Carbon Steels Figure 13.4 Temperature and Concentrations of Caustic Soda that Require Stress Relief to Prevent SCC of Carbon Steel 106 Materials Selector for Hazardous Chemicals ilarly, the presence of carbon or carbides increases the risk of this type of intergranular SCC. These conclusions are probably applicable to other low-alloy steels.21 The use of low-alloy steels is not advised under dynamic loading. Slow-strainrate laboratory tests have indicated that physical cracks in the passive layer do not heal sufficiently quickly and that hydrogen absorbed into the freshly exposed surface can cause embrittlement.22 Stainless Steels All the standard stainless steels are resistant to general corrosion by all concentrations of caustic soda up to about 65C. If low levels of iron are required in the product, then more resistant alloys or coatings may be needed. The resistance to general corrosion in caustic is almost directly proportional to nickel content. Stainless steels, however, are subject to SCC at certain concentrations and temperatures. All stainless steels can be classified into three groups according to metallurgical structure and response to heat treatment. These are the martensitic, ferritic, and austenitic groups. Further subdivisions include duplex alloys with austenitic/ferritic microstructures and precipitation-hardening (PH) grades strengthened by an age-hardening treatment. Of the conventional groups of stainless steels, the standard martensitic and ordinary ferritic grades find little application in caustic soda service. However, certain austenitic, duplex, and superferritic grades fill an economic niche at certain intermediate parameters of concentration and temperature. Ferritic Grades Ferritic stainless steels show a transition from ductile to brittle behavior over a narrow temperature band. This transition can occur above room temperature in steels with high levels of carbon, nitrogen, or chromium. This effect, combined with a tendency to sensitize from heating, limited the usefulness of the early ferritic stainless steels and restricted them to thin sections. The modern low-carbon, low-nitrogen grades (with or without stabilizing additions) have limited toughness and are still usually restricted to thin sections. The ferritic stainless steels are resistant and sometimes immune to chloride SCC. This type of alloy can be subject to 475C (887F) embrittlement caused by precipitation of ␣⬘ chromium–rich phase. The ferritic grades are also particularly prone to r-phase precipitation because of their high chromium and molybdenum contents. Both types of embrittlement can be removed by heating and rapid cooling. The standard ferritic grades of stainless steel such as 409-430 (S40900-S40300), 434, and 444 (S43400 and S44400) contain 11% to 17% Cr with carbon and nitrogen levels kept low to avoid embrittlement. These standard ferritic grades are not generally as resistant in caustic as the standard austenitic stainless steels. For example, in 60% NaOH at 212F (100C), grade 444 corroded at 0.61 mm/y (24 mpy), while MS-6: Ammonia and Caustic Soda 107 type 304 austenitic had a corrosion rate of 0.07 mm/y (3.0 mpy).23 Some of the standard ferritic grades are compared with the standard austenitic stainless steels in Table 13.5.10 There are now much more highly alloyed and corrosion-resistant ferritic stainless steels, commonly known as superferritics. The first of these superferritic stainless steels was based on 26% Cr, 1% Mo (S44627) and the columbium-stabilized XM-27 (S44627). These were developed to provide better resistance to chloride SCC than the austenitic 300 series. These ferritic steels have low interstitial content with high chromium and very low carbon levels. Other superferritic stainless steels are based on a 29% Cr, 4% Mo alloy, and they need low C Ⳮ N levels (i.e. less than 0.025%) to avoid intergranular corrosion caused by chromium depletion from precipitation of carbides and nitrides. Some of the current ferritics contain higher levels of C Ⳮ N and have additions of titanium or niobium as carbon/nitrogen stabilizers. Superferritic steels include AL 29-4C威 (S44735), AL 29-4-2威 (S44800), Sea-Cure威 (S44660), E-Brite威 (S44627), and Monit威 (S44635). While standard austenitic stainless steels show high rates in boiling 50% caustic, the higher alloy 20 and the superferritic grades tend to be resistant, as shown in Tables 13.624 and 13.7.25,26,27,28 The corrosion rates in all cases are unacceptably high, except for alloy 20 (N08020) and the superferritic stainless steels. In the standard 300 series stainless steels, the weld is less resistant than the base metal and the lowcarbon L grade more resistant than the normal carbon grades. A high-purity 30% Cr, 2% Mo (Shomac威 30-2) alloy was studied in hot, concentrated caustic and was found to be resistant to caustic strengths up to 50% at tem- Table 13.5 Corrosion of Stainless Steels in NaOH Solutions Type 302 304 309 310 410 430 304 316 329 21% Cr, 4% Ni, 0.5% Cu 410 302 304 NaOH Concentration (%) Temperature (C [F]) 20 22 20 20 20 20 72 (a) 72 (a) 72 (a) 72 (a) 72 (a) 73 (b) 73 (b) 50–60 (120–140) 50–60 (120–140) 50–60 (120–140) 50–60 (120–140) 50–60 (120–140) 50–60 (120–140) 120–125 (245–255) 120–125 (245–255) 120–125 (245–255) 120–125 (245–255) 120–125 (245–255) 100–120 (212–245) 100–120 (212–245) (a) Solution moderately agitated (b) No aeration Test Duration Corrosion Rate (Days) (mm/y [mpy]) 134 133 134 134 134 134 119 119 119 119 119 88 88 0.0025 (0.1) 0.0025 (0.1) 0.0025 (0.1) 0.0025 (0.1) 0.0025 (0.1) 0.0025 (0.1) 0.09 (3.7) 0.08 (3.1) 0.0025 (0.1) 0.15 (6) 0.8 (32) 0.97 (38) 1.1 (45) 108 Materials Selector for Hazardous Chemicals Table 13.6 Corrosion Rates of Stainless Steels in 50% NaOH at 140C (290F) Type mpy (mm/y) 304 (S30400) 316 (S31600) 439 (S43035) 444 (S44400) XM-27 (S44627) 29-4C (S44735) 29-4-2 (S44800) 180 120 300 290 1 1 1 (4.57) (3.05) (7.62) (7.37) (0.025) (0.025) (0.025) Table 13.7 Corrosion Rates (mpy [mm/y]) in Boiling 50% NaOH Solution Alloy 304 (S30400) 304L (S30403) 316 (S31600) 316L (S31603) 317L (S31703) 20 (N08020) AL 29-4C (S44735) AL 29-4-2 (S44800) E-Brite威 (S44627) Base Metal Weld 118 (3.0) 71 (1.8) 123.6 (3.1) 77.6 (1.97) 32.8 (0.83) 7.2 (0.18) 0.4 (0.01) 0.1 (0) 0.11 (0.003) 130 (3.3) 87 (2.2) 136.8 (3.5) 85.4 (2.17) 31.9 (0.81) 6.0 (015) — — — peratures below 120C (248F) under isothermal conditions. In more concentrated solutions at higher temperatures, most specimens suffered from intergranular attack (IGA). Under heat transfer conditions, corrosion was more severe, and IGA was seen even at lower caustic concentrations. The cause of IGA was found to be carbide or nitride precipitation at grain boundaries leading to chromium depletion locally. This mechanism was analogous to that occurring in austenitic stainless steels, so the same test methods for susceptibility can be applied. Galvanic coupling to more active metals prevented IGA, but potential must be strictly controlled to avoid active dissolution.29 The ferritic alloys E-Brite威 (S44627); 21/4% Cr, 1% Mo (K21950); type 446 (S44600); and 26-1S (S44626) were included in tests to determine corrosion and caustic SCC in high-temperature NaOH solutions.30 It was found that in dearated 50% NaOH at temperatures in the range 284C to 332C (543F–630F), these ferritic alloys were severely corroded, and their susceptibility to caustic SCC was dependent on their heat treatment. Superferritic alloys are sometimes used for heat exchanger tubing. They have also been successfully used in caustic evaporators but require a sustained supply of oxidizing contaminants, such as chlorates, to maintain passivity at these higher con- MS-6: Ammonia and Caustic Soda 109 centrations and temperatures. The high-purity 26-1 grade (S44626) containing niobium and molybdenum is useful up to about 175C (350F) in caustic evaporators, depending on the caustic, chloride, and other contaminant concentration.6 This type of ferritic stainless steel has been generally successful in evaporators, but failures have occurred from either localized or general corrosion. These failures have been associated with one or more of the following factors:12 • Increase in carbon levels in the alloy due to pickup from oil, grease, or other hydrocarbon contamination during tube preparation • Temperature in the first-effect evaporator 150C (300F) • High local tube temperatures caused by blockages with insoluble salts It has been shown that environmentally assisted cracking (EAC) in caustic is related to hydrogen formation so that conditions that encourage hydrogen permeation also encourage caustic cracking. Type 410 martensitic steel was found to be immune to cracking in concentrated, deaerated caustic soda solution up to 90C (194F).31 Precipitation-Hardening Grades The precipitation-hardening (PH) stainless steels see limited service in caustic environments. These alloys exhibit very high strengths combined with good notch toughness and corrosion resistance. For this reason, they are often the preferred material for such parts as valve stems and certain critical fasteners. Some of these alloys are susceptible to hydrogen embrittlement in corrosive or hydrogen-rich environments. Corrosion rates are acceptable in concentrated caustic at moderate temperatures as shown in Table 13.8.32 At the boiling point in 50% caustic, this martensitic alloy 17-4PH (S17400) in condition H 1075 was severely corroded. Tests were carried out for five 48-hour periods. Table 13.8 Corrosion Rates of 17-4PH (S17400) in Hot, Concentrated Caustic Concentration (%) 30 50 30 50 Temperature (C [F]) Corrosion Rate (mm/y [mpy]) 80 (176) 80 (176) Boiling Boiling 0.18 (7.1) 0.10 (3.9) 0.28 (11.0) 14.2 (559) Duplex Grades Duplex stainless steels have a controlled balance between austenite- and ferritebearing constituents. The “duplex” structure contains approximately 50/50 austen- 110 Materials Selector for Hazardous Chemicals ite and ferrite phases, resulting in higher strength as compared to the 18-8 grades, as well as improved corrosion resistance in some environments (e.g. chloridebearing aqueous solutions). Duplex stainless steels are not commonly used in caustic soda applications except where their resistance to chloride SCC is useful from the water side of heat exchangers. The modern grades, such as alloy 2205 (S31803), typically also contain molybdenum to achieve a composition of about 22% Cr, 5.5% Ni, 3% Mo, 0.03% C max., strengthened and stabilized by nitrogen additions. The mixed austenite-ferrite structure imparts strength and resistance (but not immunity) to chloride pitting and SCC. They have a higher strength than the lower austenitic grades, such as type 304 (S30400), but are subject to temper embrittlement at about 475C (885F). Corrosion testing in a range of caustic solutions at the boiling point showed that duplex alloys can be used in boiling solutions up to at least 30% with negligible corrosion (see Figure 13.5).33 These tests also showed that these duplex alloys were not susceptible to SCC in boiling caustic solutions from 20% to 70%. Corrosion rates of duplex stainless steels (21% Cr, 6.6% Ni, 2.3% Mo; Uranus威 50 and 26% Cr, 5.6% Ni, 3.2% Mo; Ferralium威 255) in boiling caustic soda up to 30 mass % was less than 0.01 mm/y (0.39 mpy) with uniform corrosion rates. The rate increased with caustic concentration reaching a maximum at 60 mass %, and in these more concentrated solutions, the austenitic phase was slightly more attacked than the ferritic phase. Small flow rates (e.g. 0.08 m/s rotating disc) increased the corrosion rate by a factor of six compared with stagnant conditions. Further increases in flow rate up to 1 m/s had no further effect on corrosion rate.34 Welding tends to lead to variations (20%–80%) in the austenite/ferrite ration in the as-cast weld bead and fusion zone. To minimize this effect, they are welded with a nickel-rich, overmatching rod whose higher nickel content is beneficial in caustic service. E-Brite威 and 7-Mo威 stainless steels showed good resistance to SCC and corrosion in 50% caustic at 135C (275F).6 Recent corrosion testing of various duplex stainless steels in 30% to 70% NaOH solutions found that their resistance was in the order of S32304 S32205 S32750 S32906 alloy X (a proprietary duplex alloy) Alloy 200 (N02200) had a lower corrosion rate than any of the duplex alloys in pure caustic solutions at any test temperature. Statistical analysis of the results showed that the most important alloying elements were chromium, nitrogen, and nickel to produce resistance to caustic corrosion in the duplex alloys. The effect of chromium in the austenite phase was investigated in a set of experimental duplex alloys. It was found that corrosion resistance was directly proportional to the chromium content. The range of chromium contents was from 26.54% to 29.04% with corrosion rates in boiling 60% NaOH of 0.39 mm/y to 0.05 mm/y, respectively. SCC tests in 50% NaOH at 137C (279F) showed that S32906 was immune to cracking in this environment.35 MS-6: Ammonia and Caustic Soda 111 Figure 13.5 Corrosion Resistance of Duplex Stainless Steels in Boiling NaOH Solutions Because of the uncertainties about the amount of chlorides and oxidizing salts, such as hypochlorites and chlorates, extensive field corrosion tests should be made before selecting duplex stainless steels for caustic soda service. Austenitic Grades The austenitic stainless steels constitute a large, diverse body of alloys developed from the original 18% Cr, 8% Ni stainless steel, type 302 (S30200). The standard commercial grade is type 304 (S30400), approximately 18% Cr, 8% Ni, 0.08% C. Additions of titanium or columbium in amounts equal to 5 or 10 times the carbon content, respectively, protect against sensitization and chromium carbide precipitation by precipitating carbon as titanium or columbium carbides, thus preventing chromium depletion. Types 321 (S32100) and 347 (S34700) are the common stabilized grades. A low-carbon (0.03% C max.) version is available and is widely used. Type 304L (S30403) overcomes the problems associated with chromium carbide precipitation and chromium depletion. A molybdenum-containing variant is type 316L (S31603), containing about 17% Cr, 12% Ni, and 2% to 3% Mo. The molybdenum addition improves corrosion resistance in many environments, such as in chloridecontaining solutions. There is also a stabilized version of the molybdenum-containing grade that is commonly used in Europe and is becoming more common in North America. This 112 Materials Selector for Hazardous Chemicals grade, 316Ti (S31635), is a high-carbon stainless steel with the carbon stabilized by the addition of a titanium addition equal to at least five times the carbon content. Its main application is in situations where it is exposed to temperatures between 550C and 800C (1022F and 1471F) for prolonged periods. It is used in Europe for pipelines or heated tanks handling caustic soda solutions.36 Modern steelmaking practice has made it possible to produce L grade stainless steels with controlled nitrogen additions that have the mechanical properties of the equivalent high-carbon, non–L grade. Dual grade stainless steels, 304/304L and 316/316L, are now available and permitted for use in many applications. ASME has ruled that a dual grade steel may use the straight non–L grade allowable stresses for all forms up to 540C. If this trend continues, the incidence and likelihood of intergranular attack from carbide precipitation will diminish, and demand for 321/ 347 will probably decrease further. Improved steelmaking control also means that 316 can be made more cheaply with the molybdenum content at the low end of the permitted range of 2% to 3%. This can have a serious effect on the corrosion resistance of this type of steel. In applications where molybdenum is a key factor in corrosion resistance, a minimum level should be specified, or type 317 or 317L, with 3% to 4% Mo, should be used.37 There is little difference in the corrosion resistance of types 304L (S30403) and 316L (S31603) in 50% or 73% caustic solutions. No difference was found between the corrosivity of diaphragm cell caustic and mercury cell caustic in an extensive survey of caustic soda producers.38 Corrosion of 18-8 steels in hot, concentrated caustic solutions was found to increase under heat transfer conditions, possibly due to localized evaporation and erosion of the passive film by gas bubbles. The molybdenum-containing 316 (S31600) was found to be less resistant than type 304 (S30400). Annealed and sensitized specimens were subject to IGA when held in the passive region. When in the active region, attack was general and transgranular.39 All stainless steels resist general corrosion by all concentrations of caustic soda up to about 65C (150F). Other authorities give higher temperature limits for, for example, 50% caustic, but these are probably influenced by other factors, such as the presence of impurities in the caustic. Types 304 and 316 show low corrosion rates in boiling caustic at concentrations up to nearly 20%. Type 316 has a better resistance to pitting than type 304 in caustic solutions, especially if chlorides are present. The low-carbon grades perform marginally better than the high-carbon grades because of their resistance to sensitization. This means that 316L is a good choice for caustic solutions as long as operating conditions are such that caustic SCC is not a problem.6 SCC of 304 and 316 grades occurs at around 100C (212F). An isocorrosion curve in mpy for these grades is shown in Figure 13.6.40 This figure also shows the temperature and concentration region where caustic SCC of these steels is likely. The resistance to caustic SCC in 30% NaOH at 200C increased in the order N08904 (least resistant) S31603 F138 S31803 (most resistant). (F138 is a special grade of S31603 with lower levels of inclusions.) In this environment, the addition of sulfide (20 g/L Na2S • 9H2O) dramatically increased the susceptibility of all these alloys to SCC.41 Slow-strain-rate tests in the same environment determined that the order of resistance for the alloys tested was as follows: MS-6: Ammonia and Caustic Soda 113 Figure 13.6 Isocorrosion Curves (in mpy) for 304 and 316 Stainless Steels in Caustic Soda also Showing Limits of SCC N08904 N08825 N08028 R20033 Resistance increased as the chromium content of the alloy increased. When sulfide ions were added, R20033 showed excellent corrosion resistance to pure caustic soda but was very prone to SCC even at very low strain rates.42 The zone of concentration and temperatures in which SCC is possible for 904L (N80904) is shown in Figure 13.7.43 This figure also shows the isocorrosion curve at a 0.1-mm/y corrosion rate for this and other stainless steels and for titanium. Caustic cracking can also be caused by potassium hydroxide. A type 304L (S30403) bypass line in a steam methane reforming unit failed by SCC in KOH that was formed from a potassium-promoted catalyst. Cracking at relatively low temperatures was possible only in the presence of hydrogen and/or carbon monoxide. This study found that NaOH caused cracking at lower temperatures than did KOH.44 Cast Stainless Steels Cast stainless steels perform well in caustic soda, and they are frequently used in pumps and valves. While corrosion rates are similar to those of their wrought equiv- 114 Materials Selector for Hazardous Chemicals Figure 13.7 Isocorrosion Curve for a Corrosion Rate of 0.1 mm/y for 904 L (N80904), other Stainless Steels, and Titanium alents, the presence of small amounts of ferrite in the cast “austenitic” stainless steels improves their resistance to caustic SCC compared with the fully austenitic wrought alloys.45 Cast stainless steels are often used at operating conditions well in excess of those of the wrought version.6 The equivalent cast version of types 304 (S30400) and 304L (S30403) are CF8 (J92600) and CF3 (J92700), respectively, and they exhibit approximately the same corrosion response as the wrought alloys. Isocorrosion curves for CF 8 (J92600) produced in tests at atmospheric pressure in open containers and at equilibrium pressure in closed containers are shown in Figure 13.8.46 Castings can, however, have surface layers containing more than the maximum allowable carbon content of 0.08%, which can significantly reduce corrosion resistance of the surface. In the cast form, the difference between CF 8 (J92600) or CF 3 and the corresponding molybdenum-bearing grades CF 8M (J92900) and CF 3M (J92800) is insignificant. CF 3 or CF 8 grades are not particularly common, and manufacturers of cast pumps and valves tend to standardize on CF 8M, which has a broader range of applications. The molybdenum grade castings are often more available and cheaper than the 304 or 304L grade equivalents. Since the cast version of these alloys is unlikely to be welded, there is rarely a justification for specifying the low-carbon grades in this case. This assumes that the valves or pumps, if weld repaired by the manufacturer, are properly reheat treated (solution annealed) to restore optimum corrosion resistance. Availability and price are likely to favor the non–L grade, and a properly heat-treated casting in CF 8 or CF 8M is likely to be as corrosion resistant as its low-carbon cast or wrought equivalents. MS-6: Ammonia and Caustic Soda 115 Figure 13.8 Isocorrosion Curves for CF 8 (J92600) in NaOH at Equilibrium Pressure (left) and Atmospheric Pressure (right) High-Performance Austenitic Alloys There is a group of high-performance austenitic alloys, sometimes called corrosionresistant alloys (CRAs) or superaustenitic alloys, that consists of both stainless steels (i.e. containing 50% iron) with high chromium and nickel content and alloys with nickel as the predominant alloying element. The latter group are not true nickelbased alloys because they contain 50% nickel. The original high-performance austenitic alloys were the molybdenum-free alloy 800 (20% Cr, 30% Ni; N08800) and the molybdenum-containing alloy 20Cb3威 (20% Cr, 33% Ni, 2.5% Mo; N08020). Molybdenum is now a common alloying element in this group of superaustenitic alloys, which started with the stainless steel alloy 254SMO (20% Cr, 18% Ni, 6% Mo; S31254). The molybdenum in this type of alloy gives resistance to chloride pitting, crevice corrosion, and chloride SCC. Alloy 800 (N08800) has been used in up to 73% caustic at 120C (250F) but is susceptible to caustic SCC above 150C (300F). Alloy 825 (N08825), which has 3% Mo, is slightly more resistant than alloy 800 (N08800). These nickel-chromium-iron alloys with and without molybdenum (e.g. N08800, N08825, N08020) have useful resistance up to 73% caustic at temperatures up to about 120C (250F). The beneficial effects of nickel and molybdenum in nickel-containing and nickel-based alloys is shown in Figure 13.9.47 These data show that both these alloying elements improve corrosion resistance in this caustic solution. The effect of molybdenum is minimal above about 3% to 4% Mo. 116 Materials Selector for Hazardous Chemicals Figure 13.9 Effect of Molybdenum and Nickel in Various Alloys on Corrosion Rate in NaOH Solutions In studies of austenitic and duplex alloys in 30 wt% NaOH at 150C, it was found that under deaerated conditions, chromium and nickel passivate the alloys and that iron is dissolved. Under very oxidizing conditions, chromium was dissolved, probably as CrVI, and that nickel and iron form a thicker, less protective oxide film. Caustic SCC cracking occurred in alloy 800 (N08800) only at high anodic potentials where chromium is being dissolved from the oxide.48 In oxygenated 50% caustic at 300C (572F), an increase in nickel content between 15% and 45% was found to improve the caustic SCC resistance of iron-nickelchromium alloys containing 10% to 15% chromium but had little effect on alloys with 20% to 25% chromium.49 Nickel also has a beneficial effect on the corrosion resistance of iron-based alloys in hot caustic solutions. The higher the nickel content, the lower the corrosion rate, as shown in Figure 13.10.50 The maximum benefit is reached at about 20% nickel with little further benefit from further increases in nickel into the nickel-based alloys. These data also show that when the caustic was deaerated (with hydrogen), the corrosion rates for all these metals and alloys were reduced. Comparative tests of various stainless steels in 50% NaOH are shown in Table 13.9.51 This shows the lowest temperature at which the corrosion rate exceeds 5 mpy (0.127 mm/y). These same data are plotted for the three alloying elements in Figure 13.11. Nickel has a strong beneficial effect on corrosion resistance, while there is no apparent benefit of additional molybdenum or chromium in this environment, within the range of compositions represented by these steels. MS-6: Ammonia and Caustic Soda 117 Figure 13.10 Effect of Nickel Content on Corrosion in NaOH of Various Alloys With and Without Oxygen in the Atmosphere Table 13.9 Lowest Temperature at Which Corrosion Rate Exceeds 5 mpy (0.127 mm/y) Alloy % Ni % Cr % Mo Temperature (F [C]) 304 316L 2205 Code Plus Two SAF 2304 (S32304) SAF 2507 254 SMO (S31254) 654 SMO (S32654) 904L (N08904) 9 13 5 4.5 7 18 22 25 18.5 17.5 22 23 25 20 24 20 — 2.7 3.2 — 4.0 6.1 7.3 4.5 185 (85) 194 (90) 194 (90) 203 (95) 230 (110) 239 (115) 275 (135) Boiling (142C) Data on the corrosion of the 6% Mo austenitic stainless steel alloy 6XN (N08367) and other steels and alloys in boiling 50% NaOH are shown in Table 13.10.52 These data show that none of these materials is really acceptable for this duty. For pumps and valves, a cast version of the “20” type alloy CN7M (20% Cr, 29% Ni, 3% Mo; N08007) is quite often employed. Figure 13.12 shows an isocorrosion chart for this alloy up to 73% NaOH and compares it with a cast duplex stainless steel (CD-4MCu).53,54 Other isocorrosion curves for this alloy in caustic soda are shown in Figure 13.13.55 The two sets of data in this figure represent results at atmospheric pressure and also in a closed container. The corrosion rates in the closed 118 Materials Selector for Hazardous Chemicals Figure 13.11 Effect of Molybdenum, Nickel, and Chromium on the Threshold Temperature at Which the Corrosion Rate Exceeds 5 mpy (0.127 mm/y) in 50% NaOH Table 13.10 Corrosion Rates (mpy [mm/y]) for Various Alloys in Boiling 50% Sodium Hydroxide Type 316L 77.69 (1.92) Type 317L 32.78 (0.83) Alloy 904L 9.61 (0.24) Alloy AL-6XN 11.4 (0.29) Alloy 276 17.77 (0.45) container, that is, at temperatures above the boiling point, are substantially higher than those at lower temperatures. Corrosion rates are below 0.13 mm/y (5 mpy) for the complete range of NaOH concentration at temperatures up to the atmospheric boiling point. Alloy 28 (N08028) is another iron-nickel-chromium alloy that has useful resistance to caustic solutions, particularly if contaminated. This alloy is compared with alloy 800 (N08800) and alloy 904L (N08904) in NaOH with and without chlorides in Table 13.11.56 These data show that the presence of chlorides does not significantly increase corrosion in these alloys; in fact, it decreases the corrosion of alloy 28 and alloy 800 in the 43% caustic. Time of tests was a series of 1 Ⳮ 3 Ⳮ 3 days. An isocorrosion curve for alloy 28 (N08028) based on laboratory and plant tests together with service experience is shown in Figure 13.14.56 This figure also includes comparative data for 304L (S30403) and 316L (S31603) and shows that this ironchromium-nickel-molybdenum alloy is much more resistant in hot, concentrated caustic than the standard stainless steels. MS-6: Ammonia and Caustic Soda Figure 13.12 Isocorrosion Data for CN 7M (J92700) Compared with CD 4MCu (J93370) in NaOH Solutions Figure 13.13 Isocorrosion Curves for CN 7M (J92700) in NaOH at Equilibrium Pressure (left) and Atmospheric Pressure (right) 119 120 Materials Selector for Hazardous Chemicals Table 13.11 Corrosion Rates (mm/y [mpy]) of Various Alloys in NaOH and NaOH/ NaCl Alloy 28% NaOH 28% NaOH/8% NaCl 43% NaOH 43% NaOH/6.7% NaCl (99C [210F]) (99C [210F]) (135C [275F]) (135C [275F]) Alloy 28 0.008 (0.31) Alloy 800 0.011 (0.43) 904L 0.013 (0.51) 0.008 (0.31) 0.013 (0.51) 0.018 (0.71) 0.074 (2.91) 0.397 (15.63) 0.301 (11.85) 0.045 (1.77) 0.283 (11.14) 0.349 (13.74) Figure 13.14 Isocorrosion Curves (in mm/y) for Alloy 28 (N08028) and Standard Austenitic Stainless Steels in NaOH Solutions The effect of nickel on the corrosion resistance of iron-chromium-nickel alloys is shown in Figure 13.15.57 These results of tests in 28% NaOH with 8% NaCl at 99C (210F) show that nickel-free and high-nickel alloys are resistant with a maximum corrosion rate at about 5% nickel. The filled dots in this figure relate to alloy 28 (N08028), which has good resistance in this chloride-contaminated environment. This alloy has been used for the evaporation of diaphragm cell NaOH. In this application, erosion by sodium chloride crystals can be a problem, and this alloy showed considerably better performance than pure nickel. Alloy 33威 (R20033) is an austenitic alloy based on chromium with a nominal analysis of 33% Cr, 32% Fe, 31% Ni, 1.6% Mo, 0.6% Cu, and 0.4% N. Testing in caustic soda has shown that it could be used to replace standard austenitic stainless steels in, for example, pipes or vessels for hot caustic solution where type 316Ti MS-6: Ammonia and Caustic Soda 121 Figure 13.15 The Effect of Nickel on the Corrosion of Iron-Nickel-Chromium Alloys in Caustic Soda (S31635) is limited to 90C (190F). This alloy 33 could be acceptable in 25% and 50% NaOH up to the boiling point based on 28-day laboratory tests (see Table 13.12).36 At temperatures of the order of 300C (575F), even 25% caustic will cause SCC of the CRAs. It is very important, when both chlorides and caustic are present in an environment causing SCC, to know which is the active species. If it is the chloride, a CRA will suffice to overcome the problem, whereas if caustic is the cause of the cracking, then a high-nickel alloy will be needed. Nickel and Its Alloys The nickel-based alloys are generally to be preferred for caustic soda service but are not without their problems. They may be discussed under separate groupings that distinguish between the chromium-free alloys (resistant to reducing corrosive conditions) and the chromium-bearing alloys, which are more resistant under oxidizing conditions. Nickel and nickel alloys can suffer from caustic SCC. The range of conditions that can cause SCC in nickel and various nickel alloys and stainless steels is shown in Figure 13.16. These data show that nickel and the high-nickel alloys are susceptible to SCC only in very hot, concentrated caustic and that alloy 600 is more resistant than nickel.4 Nickel, alloy 400, alloy 600, and alloy 690 are all susceptible to SCC over a wide range of caustic concentrations at temperatures above 290C (550C).58 122 Materials Selector for Hazardous Chemicals Table 13.12 Corrosion Rates (mm/y [mpy]) of Stainless Steels in Sodium Hydroxide at Various Conditions 25% NaOH Alloy 316 Ti X1CrNiMoN 25-25-2 33 50% NaOH 75C (167F) 100C (212F) BP 104C (189F) 75C (167F) 100C (212F) 125C (257F) BP 146C (333F) 0.01 (0.39) 0.01 (0.39) 0.01 (0.39) 0.12 (4.72) 0.03 (1.18) 0.01 (0.39) 0.63 (24.8) 0.02 (0.79) 0.01 (0.39) 0.08 (3.15) 0.01 (0.39) 0.01 (0.39) 0.35 (13.8) 0.01 (0.39) 0.01 (0.39) 1.60 (63) 0.26 (10.2) 0.01 (0.39) 7.99 (315) 1.35 (53) 0.01 (0.39) Figure 13.16 SCC Regions for Nickel and Other Alloys in NaOH Chromium-Free Alloys Commercially pure nickel, alloy 200 (N02200), is one of the best metals for resisting corrosion while simultaneously avoiding unacceptable metal contamination. Alloy 200 has a corrosion rate of 0.2 mpy (0.005 mm/y) below 50% NaOH at up to boiling temperature. Above 315C (600F), the low-carbon variant, alloy 201 (N02201), must be used to avoid embrittlement by graphitization and attendant MS-6: Ammonia and Caustic Soda 123 intergranular attack.59 A chart showing corrosion regions for alloy 200 and 201 is shown in Figure 13.17.7 The mechanical strength of nickel also diminishes at elevated temperatures, encouraging the use of chromium-bearing nickel alloys. Nickel has corrosion rates of 0.1 mpy (0.0025 mm/y) up to 73% NaOH at 115C (240F) and only 1 mpy (0.025 mm/y) at 130C (265F). Caustic soda is usually produced at 11% to 15% solution and concentrated up to 50% or higher by evaporation in nickel 200 vessels. It has outstanding corrosion resistance to caustic soda at all concentrations up to anhydrous at boiling or molten temperatures.6 Nickel is more severely attacked in hot, concentrated caustic under heat transfer conditions and in the presence of chlorates in solution.60 The presence of oxidizable sulfur compounds also increases the corrosion of nickel in caustic. The effect is more pronounced with sulfides, such as hydrogen sulfide, mercaptans, or sodium sulfide, and, to a lesser extent, with partly oxidized compounds, such as thiosulfates and sulfites.59 Alloy 201 (N02201) and alloy 33 (R20033) were exposed in caustic evaporators in various Russian plants and to laboratory evaluation. This study concluded the following:61 • Alloy 201 and its welded joints were resistant to corrosion in evaporation of electrolytic caustic soda solutions (from both mercury and diaphragm cells) up to 60% NaOH at temperatures up to 170C; increased grain growth (index 2–3) was Figure 13.17 Isocorrosion Curve (in mpy) for Alloy 200 (N02200) and Alloy 201 (N02201) in Caustic Soda 124 Materials Selector for Hazardous Chemicals seen in the HAZ of the welded joints of alloy 201, which may be a contributing factor to the failure of the welded joint of the alloy 201 in diaphragm caustic soda in more severe conditions: 65% to 70% NaOH, 180C to 195C (365F–383F). Intercrystalline and knife-line (IGA) corrosion was seen in the welded joint, and the weld metal suffered selective corrosion. The overall corrosion rate of the welded joint of alloy 201 was 0.8 mm/y. • The base metal and welded joints of alloy 201 were fairly corrosion resistant when concentrating to more than 60% NaOH of mercury cell caustic soda; alloy 33 and its welded joints possess good resistance under the conditions of evaporation concentration of diaphragm cell caustic soda (up to 65% NaOH), but, as the concentration increased to 70%, the corrosion rate of the welded joint increased to 0.3 mm/y with general corrosion and marked etching of the weld metal; at higher concentrations of the diaphragm cell caustic soda, the alloy suffered pitting corrosion. • Under the conditions of concentration from 45% to 60% of mercury cell caustic soda (free from chlorides), alloy 33 was resistant, but not as resistant as alloy 201 (0.019 and 0.0065 mm/y, respectively). At caustic soda concentrations rising from 60% to 97.5%, the corrosion rate of alloy 33 was about one-third that of alloy 201 (0.125 and 0.336 mm/y, respectively). On further concentration of the caustic soda to 99.5%, the corrosion rate of alloy 201 was about 20 times higher than that of alloy 33 (0.0022 and 0.0485 mm/y, respectively). When even nickel ion contamination must be minimized, cathodic protection may be applied in concentrators and storage tanks, with effective current densities as low as 0.11 A/m2 (0.01 A/ft2).14 The corrosion rate increases linearly with chlorate contamination and other oxidizing contaminants. The behavior of alloy 201 (N02201) is compared with other nickel alloys in molten caustic in Table 13.13.62 It can be seen that nickel is more resistant to corrosion in molten caustic than the more highly alloyed materials even at elevated temperature. In molten caustic containing 0.5% sodium chloride, 0.5% sodium carbonate, and 0.0.03% sodium sulfate at 510C (950F), the corrosion rate of wrought nickel was 9 mpy (0.23 mm/y). This high rate was still much lower than rates measured for other alloys tested.10 Rates of over 50 mpy (1.27 mm/y) were determined for nickel in laboratory tests in 75% caustic concentrated to the anhydrous product at 480C (900F). This rate was also much lower than the other alloys tested, apart from silver, which corroded at a rate of 5.3 mpy (0.135 mm/y).63 In plant exposures at 540C Table 13.13 Static Corrosion Rates (mpy [mm/y]) in Molten Caustic Soda Alloy (UNS No.) Alloy 201 (N02201) Alloy 400 (N04400) Alloy 600 (N06600) 400C (750F) 500C (932F) 0.9 (0.022) 1.8 (0.046) 1.1 (0.028) 1.3 (0.033) 5.1 (0.13) 2.4 (0.061) 580C (1076F) 680C (1256F) 2.5 (0.064) 17.6 (0.45) 5.1 (0.13) 37.8 (0.96) — 66.4 (1.69) MS-6: Ammonia and Caustic Soda 125 (1000F), the corrosion rate of nickel soared to 260 mpy (6.60 mm/y) because of sulfur and nitrate contamination.64 The least expensive chromium-free, nickel-based alloy is alloy 400 (N04400), and it is stronger than nickel itself. It is used in caustic soda service up to about 73% but, having a 25% to 30% copper content, may be objectionable from the contamination standpoint in some applications where it might be suitable from the corrosion point of view. It is less resistant than nickel at concentrations above 73% at the atmospheric boiling point and can suffer SCC in hot caustic. It is subject to SCC at higher temperatures, such as in 0.2 to 0.27 MPa (300–400 psi) steam contaminated with alkaline boiler-treating chemicals. Also, liquid metal embrittlement (LME) has occurred with alloy 400 (N04400) components handling mercury cell caustic. Alloy K-500 (N05500), a tougher and more abrasion-resistant variant that undergoes precipitation hardening, has substantially the same corrosion characteristics as alloy 400. Results of plant tests of various nickel-based alloys and other materials in caustic evaporators are shown in Table 13.14.59 These data show the superiority of nickel over the other alloys but also show that alloy 400 and alloy 600 have acceptable corrosion resistance. Nickel-clad steel plate is often used to provide simultaneously the corrosion resistance of nickel and the strength of steel at moderately elevated temperatures. Of course, to prevent corrosion of the substrate and iron contamination, special precautions must be taken in fabricating and welding. Table 13.14 Corrosion Rates (mpy [mm/y]) of Nickel and Other Materials in Caustic Evaporation Plants 14% NaOH First-Effect Multieffect Evaporator Alloy Alloy 200 Alloy 400 Alloy 600 Mild steel Cast iron NiResist威 type 1 NiResist威 type 2 3% Ni cast iron Copper 75% Cu, 20% Ni, 5% Zn 14% Cr steel 23% NaOH in Tank Receiving Liquor from Evaporator Single-Effect Evaporator— NaOH from 30% to 50% Ave. temp. 190F Ave. temp. 220F Ave. temp. 179F (88C) (104C) (81.7C) 0.02 (0.001) 0.05 (0.001) 0.03 (0.001) 8.20 (0.21) 8.20 (0.21) 2.90 (0.07) — — — — — Evaporator Concentrating NaOH to 50% — 0.16 (0.004) 0.20 (0.005) 0.17 (0.004) — — — — — — — 0.10 (0.003) 0.19 (0.005) — 3.70 (0.09) 7.00 (0.18) — — — 2.30 (0.06) 0.50 (0.01) 0.1 (0.003) — 0.3 (0.008) — 22 (0.56) 4 (0.10) 2.6 (0.07) 9 (0.23) — — — 33.0 (0.84) — 126 Materials Selector for Hazardous Chemicals The molybdenum-rich alloy B2 (N10665) has good corrosion resistance in the absence of oxidizing contaminants but finds no application because of the excellent resistance of nickel itself. Although electroless nickel plating (ENP) has a corrosion resistance superior to pure nickel 200 (N02200), such coatings are used only to minimize iron contamination since they contain “holidays,” which allow the caustic solution to access the steel surface. The resistance of ENP in caustic depends on its phosphorus content. The phosphorus is present since ENP is produced by the autocatalytic reduction of nickel in the presence of sodium hypophosphite. In tests of ENP samples with various phosphorus contents (from 0 to 18.2 at%) in 50% NaOH at room temperature, the following was found:65 • All coatings had a corrosion rates of ⱕ2 lm/y (0.002 mm/y) in 50% NaOH at RT. • High-phosphorus EN (HPEN) was less resistant than low-phosphorus (LPEN) or medium-phosphorus (MPEN) coatings in hot, concentrated NaOH. LPEN and MPEN had corrosion rates similar to pure nickel in this environment. • This inverse effect of phosphorus on corrosion resistance was said to be due to the formation of soluble nickel complexes in the presence of high phosphorus leading to the removal of Ni from the film instead of the formation of the protective nickel hydroxide/nickel oxide film. Chromium-Bearing Alloys The nickel-iron-chromium alloys can be subject to caustic SCC in hot, concentrated caustic. In deaerated 50% NaOH, the presence of nickel improves the resistance to SCC. In aerated 50% NaOH, both chromium and nickel need to be present to resist caustic SCC. The presence of molybdenum in these alloys was found to have no effect on the cracking resistance in dearated 50% NaOH. Similarly, no particular heat treatment improved the resistance of these alloys to caustic SCC. The effect of temperature in the range tested, 284C to 332C (543F–630F), was found to have only a minor effect on the extent of cracking.30 The beneficial effect of nickel in weaker dearated caustic (10%) at 316C (601F) was found to be less pronounced with alloy 800, alloy 600, and alloy 690, all showing similar cracking resistance.66 The most commonly used chromium-bearing nickel alloy is the basic nickelchromium-iron alloy 600 (76% Ni, 16% Cr, 8% Fe; N06600). This alloy combines corrosion and heat resistance with excellent mechanical strength and workability. The high nickel content provides resistance to caustic corrosion. It is also resistant to chloride-induced SCC and to corrosion by many organic and inorganic acids and water should equipment be exposed to these environments external to the caustic application. Alloy 600 (N06600) is the preferred alloy for NaOH at higher temperatures, especially if sulfur compounds are present. The high chromium content of alloy 600 confers resistance to sulfur compounds and oxidizing environments in caustic. Alloy 600 (N06600) has performed well in alkaline sulfur solutions, such as those encountered in the manufacture of sulfate or kraft paper. However, sulfur contamination MS-6: Ammonia and Caustic Soda 127 of nickel alloys can cause a form of liquid metal cracking or embrittlement (LME) at welding temperatures. This is not, in fact, true LME but a form of intergranular penetration caused by the formation of a low-melting-point nickel/nickel sulfide eutectic. Alloy 600 (N06600) is more resistant to this type of attack than is alloy 201 (N02201).12 Minimal corrosion rates for alloy 600 (N06600) are found in boiling NaOH up to about 50% concentration, as shown in Figure 13.18, in comparison with alloy 201 (N02201).67,68 However, this alloy is subject to caustic SCC above about 190C (375F) in strong caustic, although short-term U-bend tests showed no cracks after 1 month in various solutions of caustic up to 70% at 184C (363F). The possibility of SCC can be lessened if the alloy is fully stress relieved for 1 hour at 900C (1650F) or for 4 hours at 790C (1450F). In tests to assess the effects of heat treatment on SCC resistance, alloy 600 (N06600) and alloy 690 (N06690) were compared in 40% NaOH at 315C. It was found that sensitized alloy 600 was more resistant to SCC than the fully annealed samples. The explanation given is that the chromium carbides present in the sensitized alloy have a more beneficial effect on SCC than the detrimental effect of chromium depletion. Effects of carbides and chromium depletion on IGA were not investigated in these tests. SCC resistance also increased with increase in grain size and with chromium content.69 Alloy 600 (N06600) is used worldwide as a steam generator tube material in pressurized light water reactor nuclear plants where caustic SCC is also encountered. In this case, the environment is typically 10% NaOH at around 300C (572F).70 Figure 13.18 Isocorrosion Curves for Alloy 600 (N06600) and Alloy 201 (N02201) in Caustic Soda 128 Materials Selector for Hazardous Chemicals A variant of alloy 600 (N06600) is alloy 601 (N06601). This solid-solutionstrengthened higher-chromium (23%) alloy was developed primarily for hightemperature applications. However, it too shows excellent corrosion resistance in up to 98% caustic because of the high nickel content. The nickel-chromium-molybdenum alloys, such as alloy 625 (N06625) and alloy C276 (N10276), are rarely employed in caustic service because the conventional nickel alloys are usually suitable. The improvement in corrosion resistance afforded by the high molybdenum content is minimal for all practical purposes in this service. Although not usually associated with alkaline service, these expensive alloys may be usefully employed or encountered in some special applications. In 10% NaOH at 199F (93C), there was no measurable corrosion on alloy 625 (N06625). In boiling 50% NaOH at 143C (289F), a corrosion rate of 0.061 mm/y (2.4 mpy) was measured on alloy 625, while on alloy C276 the corrosion rate was 0.452 mm/y (17.8 mpy).71 The other chromium-containing nickel-based alloy that is sometimes used in caustic is alloy 690 (N06690). The corrosion rate of alloy 6030 (N06690) in 70% NaOH at 170C (338F) was 0.03 mm/y (1.18 mpy). In the same tests, alloy 400 (N04400) and alloy 33 (R20033) had the same corrosion rate (i.e. 0.03 mm/y), while alloy 59 (N06059) corroded at 0.48 mm/y (18.9 mpy) and alloy C-22 (N06022) at 0.51 mm/y (20.1 mpy).72 Clamps made from an alloy of nickel with 16% Mo, 15% Cr, and tungsten (C type alloy) holding internal steam coils in a reactor failed by caustic SCC after 3 years service. The reactor environment was 20% organics, 8% NaCl, 4% NaOH, and balance water, and the steam in the coils was at 180C (356F). It was postulated that the caustic must have become more concentrated under the clamps. An extensive investigation found that while the 22% Cr and 25% Cr duplex stainless steels were not immune to caustic SCC, they were much more resistant than the original nickelbased alloy. The failed clamps were replaced with a duplex stainless steel that is still in service after more than 12 years.73 Failures of alloy C-276 (N10276) plate heat exchanger and other field failures by dealloying have been reported.2 Laboratory investigation showed that other high-performance nickel alloys, including the entire “C” family apart from alloy C4 (N06455), are susceptible to this type of attack. The effect is to remove alloying elements, such as chromium, tungsten, molybdenum, and to some extent iron, from the matrix. It was found that dealloying did not occur at test temperatures below 90C (194F) or in caustic solutions with 5% or 0.5% NaOH. Tests in reagent grade 50% caustic produced dealloying only at test temperatures of 140C (284F), and attack occurred at lower temperatures if oxidizers were present; in this, case 0.5% sodium chlorate was used. Potassium hydroxide was found to be less aggressive at causing dealloying than sodium hydroxide. Copper and Its Alloys There is very little published corrosion data on copper alloys in caustic soda. This is because the major industries that use large amounts of caustic find copper con- MS-6: Ammonia and Caustic Soda 129 tamination intolerable. For example, copper causes rancidity in soap and discoloration in rayon. Copper, gunmetal (C90550), and bronze have limited resistance to caustic soda and potash, as do copper-nickel alloys and high-tin, aluminum bronze.74 High-zinc brasses suffer dezincification and should be avoided. However, conventional bronze castings, such as the 85% Cu, 5% Sn, 5% Zn, 5% Pb (C83600), can be used for valves or pumps for 25% caustic solutions, for example. Bronzes solution annealed at 850C (1,560F) can show SCC at pH 12.3 (about 500 ppm NaOH). Corrosion resistance does increase with nickel content, and the 90-10 cupronickel (C70600) and 70-30 grade (C71500) are sometimes used for caustic service. Because of improved heat transfer properties and strength, the 70-30 (C71500) grade has been used in evaporators up to 50% concentration in applications in which copper contamination is acceptable. Copper piping has sometimes been used for caustic soda solutions in situations in which thermal stress relief of steel piping was impractical.15 In the absence of oxidizing agents (e.g. chlorites, chlorates), copper may be used up to 73% NaOH to 100C (212F). In molten caustic, however, copper is much less resistant than iron or nickel, being attacked at about 500 mpy (12.7 mm/y) as compared with 20 mpy (0.51 mm/y) and 7 mpy (0.18 mm/y), respectively, for the alternative materials. Titanium and Its Alloys Titanium (e.g. R50400) is a reactive metal that forms a thin, tenacious, self-healing oxide film in oxygen-containing environments. It resists alkaline media, including caustic solutions at subboiling conditions, but is not recommended for boiling concentrated solutions. Temperature and concentration limits for titanium are 80C (175F) and 50% NaOH. Titanium is at “high” risk for LME in caustic contaminated by mercury. Titanium is also rapidly attacked by hot caustic containing powerful oxidizing agents, such as permanganate in pulp/paper applications. Titanium and its alloys experience excessive pickup of hydrogen from corrosion reactions, causing hydride formation and possible hydrogen embrittlement in NaOH above 80C (175F) at pH of 12 or more (400 ppm NaOH).6 Corrosion of titanium increases rapidly as temperature increases; for example, the corrosion rate is 0.18 mm/y in 73% NaOH at 130C (266F) but is 1 mm/y at 190C (374F). Titanium finds application (subject to the limitations noted) chiefly where the surrounding media external to the caustic vessel or assembly contain impurities such as chloride, chlorate, hypochlorite, and wet chlorine. For example, titanium was not corroded in 10% NaOH Ⳮ 15% NaCl at 82C (180F) or in 60% NaOH Ⳮ 2% sodium hypochlorite Ⳮ trace ammonia. In 50% NaOH containing free chlorine at 38C (100F), the corrosion rate was 0.023 mm/y. The corrosion behavior of commercially pure titanium in various caustic solutions is shown in Table 13.15.75,76 In a highly alkaline mixture of 52% NaOH and 16% NH3, titanium corroded 0.9 mm/y (35.4 mpy) in long-term tests at about 140C. In 73% NaOH without am- 130 Materials Selector for Hazardous Chemicals Table 13.15 Corrosion Rates of Commercially Pure Titanium in Various Solutions of NaOH Concentration (%) 5–10 10 28 40 50 50–73 73 75 Saturated Temperature (C [F]) Corrosion Rate (mm/y [mpy]) 21 (70) Boiling Boiling RT 93 (199) 121 (250) 38–57 (100–135) 66 (151) 80 (176) 121 (250) 190 (374) 60 (140) 110 (230) 116 (240) 129 (264) 121 (250) RT 0.001 (0.04) 0.02 (0.79) 0.0025 (0.10) 0.13 (5.1) 0.064 (2.5) 0.127 (5.0) 0.013 (0.5) 0.018 (0.7) 0.0025–0.013 (0.1–5.0) 0.033 (1.3) 43 (1.09) 0.18 (7.1) 0.051 (2.0) 0.127 (5.0) 0.178 (7.0) 0.033 (1.3) Nil monia, the corrosion rate of titanium was only about 0.18 mm/y (7.1 mpy) at about 130C.77 In tests to assess the resistance of titanium and other alloys to caustic SCC at elevated temperature, it was found that the corrosion rate was too high to detect discrete cracks in the specimens. These tests were carried out in 50% NaOH at temperatures in the range of 284C to 332C (543F–630F).30 Zirconium and Its Alloys Zirconium (e.g. R60702) is a reactive metal, forming a very resilient oxide film (zirconia) that provides resistance to almost all alkalis, fused or in solution. The oxide film is self-healing and is protective up to 300C (570F). Zirconium has outstanding resistance to sodium hydroxide, tending to mirror the performance of nickel. Zirconium is considered useful in 73% NaOH at up to 138C (280F). Like titanium, zirconium is considered at high risk for LME in caustic contaminated with mercury. Zirconium is not attacked by oxidizing media unless chlorides are present.14 Zirconium is very resistant to molten sodium hydroxide at temperatures above 1000C (1830F). It is resistant to SCC in boiling caustic solutions.6 Corrosion rates of zirconium Zr702 (R60702) in pure and contaminated caustic soda show that it is resistant to all conditions except very hot, very strong solutions (see Table 13.16).78 MS-6: Ammonia and Caustic Soda 131 Table 13.16 Corrosion Rates of Zirconium (R60702) in Sodium Hydroxide Solutions and Mixtures Environment Temperature (C [F]) Corrosion Rate (mpy [mm/y]) 5%–10% NaOH 21 (70) 1 (0.025) 28% NaOH Room 1 (0.025) 10%–25% NaOH Boiling 1 (0.025) 40% NaOH 100 (212) 1 (0.025) 50% NaOH 38–57 (100–135) 1 (0.025) 50%–73% NaOH 188 (370) 20–50 (0.508–1.27) 73% NaOH 110–129 (230–1,000) 2 (0.05) 73% NaOH to anhydrous 212–538 (414–1,000) 20–50 (0.508–1.27) 9%–11% NaOH, 15% NaCl 82 (180) 1 (0.025) 10% NaOH, 10% NaCl, wet CoCl2 10–32 (50–90) 1(0.025) 0.6% NaOH, 2% NaCl, trace of NH3 129 (264) 1 (0.025) 191 (376) 1 (0.025) 7% NaOH, 53% NaCl, 7% NaClO3, 80–100 ppm NH3 52% NaOH, 16% NH3 138 (280) 5 (0.127) 20% NaOH (suspended salt, violent boiling) 60 (140) 10–20 (0.254–0.508) 50% NaOH, 750 free Cl2 38–57 (100–135) 1 (0.025) Cast pumps and valves utilize the benefits of zirconium where service conditions are too severe for stainless steels, nickel alloys, or titanium. Other Nonferrous Metals and Alloys The common nonferrous metals, such as zinc, tin, and lead, are amphoteric and find no application in caustic service. In ambient aqueous alkaline solutions, niobium has corrosion rates of less than 0.025 mm/y (1 mpy). At higher temperatures, even though the corrosion rate does not seem excessive, niobium is embrittled even at low concentrations (5%) of sodium hydroxide and potassium hydroxide. Like tantalum, niobium is embrittled in salts that hydrolyze to form alkaline solutions. These salts include sodium and potassium carbonates and phosphates.79 Silver is probably the most resistant metal in caustic soda and is not attacked by sodium hydroxides at temperatures below 500C (930F). At one time, it was used in the evaporation of anhydrous caustic. Tantalum is destroyed in caustic solutions by the formation of successive surface oxide layers. The rate of attack increases with increasing concentration or temperature, and tantalum is dissolved in molten alkalis. Tantalum has, however, been used in dilute alkaline solutions (pH 10) in a paper mill.80 Ta –10 wt% W alloy showed 132 Materials Selector for Hazardous Chemicals passive behavior in 5%, 10%, and 15% NaOH at 50C and in 5% NaOH at 75C. This tantalum-tungsten alloy was found to be slightly more corrosion resistant than tantalum when both materials were in the passive state but less resistant under active corrosion conditions. The tantalum-tungsten alloy can be used in caustic only over a very limited range of concentrations at low temperatures.81 References 1. Anon, “Aluminium with Food and Chemicals” (London, UK: Alcan Industries Ltd, 1966), p. 91. 2. M.R. Tabrizi, S.B. Lyon, G.E. Thompson, J.M. Ferguson, “The Long Term Corrosion of Aluminium in Alkaline Media,” Corrosion Science 32, 7 (1991): pp. 733–742. 3. M.L. Doche, J. Rameau, R. Durand, F. Novel-Cattin, “Electrochemical Behaviour of Aluminium in Concentrated NaOH Solutions,” Corrosion Science 41, 4 (1999): pp. 805–826. 4. M. Hagen, “Corrosion of Steels,” in Corrosion and Environmental Degradation, vol. II, ed. M. Schutze (Weinheim, Germany: Wiley-VCH, 2000), pp. 1–68. 5. S. Giddey, B. Cherry, F. Lawson, M. Forsyth, “Stability of Oxide Films Formed on Mild Steel in Turbulent Flow Conditions of Alkaline Solutions at Elevated Temperatures,” Corrosion Science 43, 8 (2001): pp. 1497–1517. 6. B.D. Craig, D.B. Anderson, eds., Handbook of Corrosion Data (Materials Park, OH: ASM International, 1997), pp. 761–790. 7. Anon, “Corrosion Resistance of Nickel and Nickel-Containing Alloys in Caustic Soda and Other Alkalies,” CEB-2 (New York, NY: International Nickel Company Inc., 1973), p. 17. 8. H.T. Angus, Cast Iron: Physical and Engineering Properties, 2nd ed. (London, UK: Butterworths, 1976), p. 314. 9. R. Covert, J. Morrison, K. Rohrig, W. Spear, “Ni-Resist and Ductile Ni-Resist Alloys,” reference book no. 11018 (Toronto, ON, Canada: NiDI, 1998), 42 pp. 10. F.L. LaQue, H.R. Copson, Corrosion Resistance of Metals and Alloys (New York, NY: Reinhold Publishers, 1963), 365 pp. 11. E. Heyn, D. Bauer, “Corrosion Resistance of Materials in Alkalies and Hypochlorites,” in Process Industries Corrosion, ed. P.J. Gegner (Houston, TX: NACE, 1975), pp. 296–305. 12. C.M. Schillmoller, “Select the Right Alloys for Caustic Soda Service,” Chemical Engineering Progress May (1996): pp. 48–55. 13. Anon, “NACE Network,” reported in MP 42, 2 (2003): pp. 82–83. 14. J.K. Nelson, “Corrosion by Alkalies and Hypochlorites,” in Metals Handbook— Corrosion, vol. 13, 9th ed., ed. J.R. Davis (Metals Park, OH: ASM International, 1987), pp. 1174–1180. 15. C.P. Dillon, Corrosion Control in the Chemical Process Industries, 2nd ed. (St. Louis, MO: MTI, 1994), 424 pp. 16. R.B. Norden, “Materials of Construction,” pp. 23–14. MS-6: Ammonia and Caustic Soda 133 17. Anon, “Corrosion by Caustic Solutions” (Houston, TX: The Hendrix Group Inc., 2002), http://www.hghouston.com/naoh.html. 18. H.W. Schmidt, P.J. Gegner, G. Heinemann, C.F. Pogacar, E.H. Wyche, “Stress Corrosion Cracking in Alkaline Solutions,” Corrosion 7 (1951): pp. 295–302. 19. A.A. Berk, W.F. Waldeck, “Caustic Danger Zone,” Chemical Engineering 57, 6 (1950): pp. 235–237. 20. Anon, Corrosion Data Survey (Houston, TX: NACE International, 1985), p. 176. 21. N. Bandyopadhyay, C.L. Briant, “Caustic Stress Corrosion Cracking of Low Alloy Iron Base Materials,” Corrosion 41, 5 (1985): pp. 274–280. 22. R. Popperling et al. (1985), in “Sodium Hydroxide Advisor,” ChemCor 6 (MTI/ NACE/ NiDI/NIST, 1992). 23. A. Sabata, W.J. Schumacher, “Martensitic and Ferritic Stainless Steels,” in CASTI Handbook of Stainless Steels and Nickel Alloys, ed. S. Lamb (Edmonton, AB, Canada: CASTI Publishing Inc., 2000), p. 144. 24. H.E. Deverell, I.A. Franson, “Practical Guide to Newer Ferritic Stainless Steels,” MP 28, 9 (1989): pp. 52–57. 25. Anon, “ATI Technical Data Sheets,” CD-ROM (Pittsburgh, PA: Allegheny Ludlum Corp., 2001). 26. Anon, “AL 29-4C,” technical data sheet no. B-51-Ed5/7.5M/793/GP (Pittsburgh, PA: Allegheny Ludlum Corp., 1982), p. 11. 27. Anon, “AL 29-4-2,” technical data sheet no. B-153-Ed 1-10M-582P (Pittsburgh, PA: Allegheny Ludlum Corp., 1993), p. 3. 28. Anon, “E-Brite Alloy,” technical data sheet no. B-150-Ed1-10M-181P (Pittsburgh, PA: Allegheny Ludlum Corp., 1980), p. 7. 29. M. Yasuda, F. Takeya, S. Tokunaga, F. Hine, “Corrosion Behavior of a Ferrtitic Stainless Steel in Hot Concentrated NaOH Solutions,” MP 23, 7 (1984): pp. 44– 49. 30. A.R. McIlree, H.T. Michels, “Stress Corrosion Behavior of Fe-Cr-Ni and Other Alloys in High Temperature Caustic Solutions,” Corrosion 33, 2 (1977): pp. 60– 67. 31. J.G. Gonzalez-Rodriguez, V.M. Salinas-Bravo, A. Martinez-Villafane, “Hydrogen Embrittlement of Type 410 Stainless Steel in Sodium Chloride, Sodium Sulfate and Sodium Hydroxide Environments at 90C,” Corrosion 53, 6 (1997): pp. 499– 504. 32. Anon, “Armco17PH Precipitation-Hardening Stainless Steel,” bulletin no. FS-11 (Butler, PA: Armco Advanced Materials Co., 1994). 33. E.-M. Horn et al. (1991), in Corrosion and Environmental Degradation, vol. II, ed. M. Schutze (Weinheim, Germany: Wiley-VCH, 2000), pp. 69–111. 34. E.-M. Horn, S. Savakis, G. Schmitt, I. Lewandowski, “Performance of Duplex Steels in Caustic Solutions,” Duplex Stainless Steels ’91 (1991): pp. 1111–1119. 35. D. Leander, “Corrosion Characteristics of Different Stainless Steels, Austenitic and Duplex, in Naoh Environment,” Stainless Steel World Conference, Maastricht, Netherlands (2003), 9 pp. 36. M. Kohler, U. Heubner, K.W- Eichenhofer, M. Renner, “Alloy 33, a New Corrosion Resistant Austenitic Material for the Refinery Industry and Related Applications,” CORROSION/95, paper no. 338 (Houston, TX: NACE, 1995), p. 14. 134 Materials Selector for Hazardous Chemicals 37. G. Kobrin, J. Lilly, J. Mac Diarmid, B. Moniz, 1998, “Stainless Steels for Chemical Process Equipment,” NiDI reprint series no. 14 038 (Toronto, ON, Canada: NiDI, 1998), 9 pp. 38. C.M. Schillmoller, “Alloy Selection for Caustic Soda Service,” NiDI technical series no. 10019 (Toronto, ON, Canada: NiDI, March 1988), 9 pp. 39. M. Yasuda, S. Tokunaga, T. Taga, F. Hine, “Corrosion Behavior of 18-8 Stainless Steels in Hot Concentrated Caustic Soda Solutions under Heat-Transfer Conditions,” Corrosion 41, 12 (1985): pp. 720–727. 40. F.L. LaQue, H.R. Copson (1963), in “Sodium Hydroxide Advisor,” ChemCor 6 (MTI/NACE/ NiDI/NIST, 1992). 41. G. Rondelli, B. Vincenti, E. Sivieri, “Stress Corrosion Cracking of Stainless Steel in High Temperature Caustic Solutions,” Corrosion Science 39, 6 (1997): pp. 1037–1049. 42. G. Rondelli, B. Vincenti, “Susceptibility of Highly Alloyed Austenitic Stainless Steels to Caustic Stress Corrosion Cracking,” Materials & Corrosion 53 (2002): pp. 813–819. 43. Anon, “Corrosion Handbook for Stainless Steels” (Sandviken, Sweden: Sandvik Steel, 1999), p. II:54. 44. S.W. Dean, “Caustic Cracking from Potassium Hydroxide in Syngas,” MP 38, 1 (1999): pp. 73–76. 45. C. Houska, “Castings—Stainless Steels and Nickel-Base,” reference book no. 11 022 (Toronto, ON, Canada: NiDI, 2001), 88 pp. 46. R.W. Monroe, S.J. Pawel, “Corrosion of Cast Steels,” in Metals Handbook—Corrosion, vol. 13, 9th ed., ed. J.R. Davis (Metals Park, OH: ASM International, 1987), p. 580. 47. R.C. Scarberry et al. (1967), in “Sodium Hydroxide Advisor,” ChemCor 6 (MTI/ NACE/NiDI/NIST, 1992). 48. T. Cassagne, P. Combrade, “Stress Corrosion Cracking of Stainless Steels in Caustic Environments: A Laboratory Study” (Florence, Italy: Innovation Stainless Steel, 1993), pp. 3.215–3.220. 49. J.E. Truman, R. Perry, “The Resistance to Stress Corrosion Cracking of Some CrNi-Fe Austenitic Steels and Alloys,” British Corrosion Journal 1 (1965): pp. 60– 66. 50. M. Yasuda, K. Fukumoto, H. Koizumi, Y. Ogata, F. Hine, “On the Active Dissolution of Metals and Alloys in Hot Concentrated Caustic Soda,” Corrosion 43, 8 (1987): pp. 492–498. 51. Anon, “SAF Type 2507,” booklet no. AP-26-12/01 (Schaumburg, IL: Avesta Polarit Inc., 2001), p. 3. 52. J.F. Grubb, ed., “AL-6XN Alloy” (Pittsburgh, PA: Allegheny Ludlum Corp., 1995), p. 38. 53. Anon, “Durcomet 100,” bulletin no. A/7d (Dayton, OH: The Duriron Co. Inc., 1981), 8 pp. 54. Anon, “Durimet 20,” bulletin no. A/1f (Dayton, OH: The Duriron Co. Inc., 1981), 6 pp. 55. R.W. Monroe, S.J. Pawel, “Corrosion of Cast Steels,” in Metals Handbook—Corrosion, vol. 13, 9th ed., ed. J.R. Davis (Metals Park, OH: ASM International, 1987), p. 579. MS-6: Ammonia and Caustic Soda 135 56. S. Bernhardsson, S. Lagerberg, C. Martensson, M. Tynell, “Corrosion Performance of a High-Nickel Stainless Alloy in the Process Industry” (Sandviken, Sweden: Sandvik, undated), 14 pp. 57. S. Bernhardsson, J. LeGrand, “Special Stainless Steels for the Chemical and Petrochemical Industry,” lecture no. S-52-65-ENG (Sandviken, Sweden: Sandvik, 1981), 15 pp. 58. G. Kobrin, “Materials Selection,” in Metals Handbook—Corrosion, vol. 13, 9th ed., ed. J.R. Davis (Metals Park, OH: ASM International, 1987), p. 328. 59. Anon, “Resistance to Corrosion,” publication no. 3M8-88 S-37 (Huntington, WV: Inco Alloys Int., 1985), p. 33. 60. M. Yasuda, F. Takeya, F. Hine, “Corrosion Behavior of Nickel in Concentrated NaOH Solutions under Heat Transfer Conditions,” Corrosion 39, 10 (1983): pp. 399–406. 61. Y.B. Danilov, A.A. Kachanov, E.K. Gvozdikova, T.É. Shepil, V.N. Khil, T.A. Balak, V.S. Gorlova, “Corrosion Studies of Alloys from Krupp VDM in Caustic Soda Production,” Chemical and Petroleum Engineering 37, 3–4 (2001): pp. 253–258. 62. Anon, “Corrosion Resistant Alloys—Specifications and Operating Data” (Temperance, MI: Rolled Alloys Inc., 2002), p. 11. 63. Anon, CEB-2 (International Nickel Company Inc., 1969), in P.J. Gegner, “Corrosion Resistance of Materials in Alkalies and Hypochlorites,” Process Industries Corrosion (Houston, TX: NACE, 1975), p. 298. 64. R.S. Sheppard et al. (1962), in P.J. Gegner, “Corrosion Resistance of Materials in Alkalies and Hypochlorites,” Process Industries Corrosion (Houston, TX: NACE, 1975), p. 298. 65. R.L. Zeller, L. Salvati, “Effects of Phosphorus on Corrosion Resistance of Electroless Nickel in 50% Sodium Hydroxide,” Corrosion 50, 6 (1994): pp. 457–467. 66. A.J. Sedriks et al. (1979), in A.J. Sedriks, Corrosion of Stainless Steels (New York, NY: John Wiley & Sons, 1979), p. 175. 67. Anon, “Nickel Alloys 200, 2201, 270, 301,” brochure no. 10M-22-79 T-15 (Huntington, WV: Special Metals Corporation, 1979), p. 17. 68. Anon, “Inconel Alloy 600,” publication no. SMC 027 (Huntington, WV: Special Metals Corporation, 2000), p. 15. 69. H.P. Kim, S.S. Hwang, Y.S. Lim, I.H. Kuk, J.S. Kim, “Effect of Heat Treatment and Chemical Composition on Caustic Stress Corrosion Cracking of Alloy 600 and Alloy 690,” Key Engineering Materials 183–187 (2000): pp. 707–712. 70. J.S. Baek, J.G. Kim, D.H. Hur, J.S. Kim, “Anodic Film Properties Determined by EIS and Their Relationship with Caustic Stress Corrosion Cracking of Alloy 600,” Corrosion Science 45, 5 (2003): pp. 983–984. 71. Anon, “Corrosion Resistant Alloys—Specifications and Operating Data” (Temperance, MI: Rolled Alloys Inc., 2002): pp. 10–14. 72. U. Heubner M. Kohler, “High-Alloy Materials for Aggressive Environments,” VDM report no. 26 (Werdol, Germany: ThyssenKrupp VDM GmbH, 1998), p. 122. 73. G. Notten, E. van den Heuvel, H. Verhoef, “Stress Corrosion Cracking Resistance of Duplex Stainless Steels in Caustic Environments,” Stainless Steel World, Duplex America 2000, paper no. DA2_023 (2000): pp. 53–59. 136 Materials Selector for Hazardous Chemicals 74. Anon, “Corrosion Resistance of Copper and Copper Alloys,” CDA publication no. 106 (London, UK: Copper Development Association). 75. Anon, “Corrosion Resistance of Titanium,” reference no. 1431531969 (Birmingham, UK: IMI Kynoch Ltd, 1969), p. 28. 76. Anon, “Corrosion Resistance of Titanium,” brochure no. TMC-0105 (Denver, CO: Timet, 1999), p. 17. 77. Anon, Dechema Corrosion Handbook, Ammonia and Ammonium Hydroxide section, CD-ROM (Frankfurt, Germany: Dechema aV, 2001). 78. Anon, “Zircadyne Corrosion Data,” bulletin no. TWCA-8101ZR (Albany, OR: Teledyne Wah Chang, 1987), 25 pp. 79. Anon, “Niobium,” reference no. NioNio-056, CD-ROM (Pittsburgh, PA: Allegheny Ludlum Corp., 2001), 42 pp. 80. M. Schussler, “Corrosion Data Survey on Tantalum” (Chicago, IL: Fansteel Inc., 1972), p. 50. 81. A. Robin, “Corrosion Behaviour of Ta-10 wt % W Alloy in Sodium Hydroxide Solutions,” Corrosion Engineering Science and Technology 38, 3 (2003): pp. 211– 217. 14 Resistance of Nonmetallic Materials Elastomers The following list (Table 14.1) represents the dry, hot air temperature limits for some elastomeric materials. Sources vary on “maximum recommended service temperatures,” and the user is advised to consult with the supplier regarding suitability for caustic service. Seals made from neoprene latex is in service in numerous transportation applications where low-temperature curing material was required. Neoprene latex has very limited resistance to hot water or to hot, dilute NaOH. Some elastomers identified here may not be available as liners. Table 14.1 Dry, Hot Air Temperature Limits for Various Elastomers Elastomer Family Hard rubber (“Ebonite”) Soft natural rubber Neoprene* (polychloroprene) Chlorinated rubber Nitrile rubber Chlorosulfonated polyethylene (CSPE) Isobutylene isoprene (butyl) Butadiene acrylonitrile (Buna-N威) Ethylene propylene diene monomer (EPDM) Silicone rubber Fluorosilicone Fluorocarbon elastomers Perfluoroelastomers Max. Temperature 80⬚C (175⬚F) 80⬚C (175⬚F) 116⬚C (240⬚F) 115⬚C (238⬚F) 150⬚C (300⬚F) 120⬚C (250⬚F) 150⬚C (300⬚F) 150⬚C (300⬚F) 175⬚C (340⬚F) 315⬚C (600⬚F) 204⬚C (400⬚F) 250⬚C (480⬚F) 327⬚C (620⬚F) *Neoprene latix is in service in numerous transportation applications where low-temperature curing material was required. Neoprene latex has very limited resistance to hot water or to hot, dilute NaOH. It must be noted that many of these elastomers are not available as liners. In any event, the maximum temperature for immersion service is usually about 80⬚C (175⬚F). 137 138 Materials Selector for Hazardous Chemicals Table 14.2 Estimated Elastomer’s Maximum Temperature (⬚C [⬚F]) in VariousStrength Solutions of NaOH Elastomer Butadiene Butyl, grade 1 (IR) Chlorsulfonated polyethylene (CSM, Hypalon威, etc.) Fluoroelastomer (FKM, Viton威 TBR-S, ETP-S, etc.) Natural rubber, hard (NR) Natural rubber, soft (NR) Nitrile (NBR, Buna-N威, etc.) Perfluoroelastomer (FFKM, Kalrez威 6375, 1050LF, etc.) Polysulfide (T) Silicone (SI) Polytetrafluoroethylene (PTFE, Teflon威, etc.) 10% 30% 50% 70% 60 (140) 60 (140) 60 (140) 60 (140) 100 (212) 100 (212) 100 (212) 100 (212) 120 (248) 120 (248) 120 (248) 120 (248) 100 (212) 100 (212) 70 (158) 50 (122) 100 (212) 100 (212) 100 (212) 100 (212) 60 (140) 60 (140) 60 (140) 60 (140) 90 (194) 90 (194) 90 (194) 70 (158) 260 (500) 260 (500) 225 (437) 225 (437) 25 (77) 25 (77) 25 (77) 25 (77) 25 (77) 25 (77) 25 (77) 25 (77) 260 (500) 260 (500) 260 (500) 260 (500) Some suggested temperature limits for elastomeric seals for immersion service in caustic soda solutions are given in Table 14.2. These data are a compilation from various sources. Testing is recommended for applications involving elastomers in specific caustic soda environments. Plastics There are areas of use in which both thermoplastic and thermoset resins find application in caustic service. They also find some application as immersion coatings, such as modified epoxy-phenolics. From a practical viewpoint, the majority of polymeric materials can withstand caustic soda solutions up to about 80⬚C (176⬚F). Careful attention needs to be given to the coating techniques or to fabrication involving polymeric materials. Factoryapplied systems can usually be expected to be more reliable than those applied onsite. Preparation of vessels to be coated should be carried out by competent companies to a recognized standard, such as NACE RP0178, “Fabrication Details, Surface Finish Requirements, and Proper Design Considerations for Tanks and Vessels to Be Lined for Immersion Service.” Transport of coated components should be in accordance with the supplier’s directives. Some plastics, such as hydrolyzable esters, are easily permeated by dilute caustic and are therefore more resistant in more concentrated (40%–50% NaOH) solutions. Thermoplastics Polyolefins are inherently resistant to caustic within their temperature limits. Highdensity polyethylene (HDPE) and polypropylene (PP) generally show better chem- MS-6: Ammonia and Caustic Soda 139 ical resistance than the low-density PE. HDPE resists all concentrations of NaOH to 60⬚C (140⬚F). It should be noted that some copolymers are subject to environmental stress cracking (ESC) and that others, such as PE/vinyl acetate (PVA) copolymers, are nonresistant because the PVA is subject to saponification. ASTM D 1693-70, 1980, provides a standard test method for evaluating ESC of ethylene plastics. Generally, PVC, in rigid form, resists alkaline solutions to about 50⬚C (122⬚F). PVC-lined FRP piping is used for caustic service, but unsupported PVC is not safe. For safety’s sake, the chlorinated polymer CPVC, which can tolerate 10% to 70% caustic at up to about 80⬚C (175⬚F), is often preferred. This limit for CPVC is not universally accepted, and some suppliers suggest much lower limits. Similarly, there are a range of limits suggested for PVDF and some other polymers. When using any polymeric materials, it is best to consult the supplier and/or test the actual polymer under service conditions. Table 14.3 shows some suggested temperature limits for thermoplastic materials in caustic service, as a consensus of several sources. Use of many of these materials is confined to linings for pipe and vessels rather than as solid construction items because of the hazardous nature of caustic soda. Fluoroplastic materials are to be preferred, although PP-lined steel pipe has also been used to handle 50% NaOH at 90⬚C (195⬚F). Recommended temperature limits for plastic lined steel pipes are given in Table 14.4.1 Thermoplastics are also used as the resistant liner in dual laminate construction in which fiberglass-reinforced plastic (FRP) is used as the reinforcing, structural element. The corrosion resistance depends on the resistance of the thermoplastic liner, although resistant resins are often used in the construction of the FRP rein- Table 14.3 Thermoplastics Maximum Temperature (⬚C [⬚F]) in Various Strengths of NaOH Plastic Acrylic Acrylonitrile-butadiene-styrene (ABS) Polyethylene (PE) Polypropylene (PP) Chlorinated polyether Polystyrene Polycarbonate Polyvinyl chloride (PVC) Chorinated polyvinyl chloride (CPVC) Polyvinylidene chloride (PVDC) Polyvinylidene fluoride (PVDF) Fluorinated ethylene-propylene (FEP) Polytetrafluorethylene (PTFE)* Note: NR ⳱ not recommended *Limit to 150⬚C as lining 10% 30% 50% 70% 25 (77) 40 (104) 60 (140) 100 (212) 60 (140) 60 (140) 25 (77) 60 (140) 80 (176) 25 (77) 60 (140) 200 (392) 220 (428) 25 (77) 40 (104) 60 (140) 100 (212) 120 (248) 60 (140) 25 (77) 40 (104) 80 (176) 25 (77) 60 (140) 200 (392) 220 (428) 25 (77) 40 (104) 60 (140) 100 (212) 120 (248) 60 (140) NR 40 (104) 80 (176) 40 (104) 60 (140) 200 (392) 220 (428) 25 (77) 40 (104) 60 (140) 100 (212) 100 (212) 60 (140) NR 40 (104) 80 (176) 40 (104) 60 (140) 200 (392) 220 (428) 140 Materials Selector for Hazardous Chemicals Table 14.4 Temperature Limits for Plastic-Lined Pipe in Caustic Soda NaOH Concentration ⬍10% 10–50% 50% ⬎50% PVDC Saran威 PP PVDF PTFE 150⬚F (65⬚C) 75⬚F (24⬚C) 75⬚F (24⬚C) — 200⬚F (93⬚C) 200⬚F (93⬚C) 200⬚F (93⬚C) 150⬚F (65⬚C) 175⬚F (79⬚C)* 125⬚F (52⬚C)* NR NR 450⬚F (230⬚C) 450⬚F (230⬚C) 450⬚F (230⬚C) 450⬚F (230⬚C) *If mercury amalgam is present, rating drops to not resistant (NR) forcement in case of permeation and leaks in the thermoplastic liner. Most common thermoplastics are used in this type of construction, and many of them are suitable for use in caustic applications (see Table 14.5).2 Thermoset Resin Materials The thermoset resins are used almost entirely in reinforced construction, with the exception of modified epoxy coatings. FRP is used for piping and equipment in caustic service but not usually for high-concentration, high-temperature service.3,4 Such applications are contradictory to some extent in that the glass reinforcement is subject to attack by even quite weak alkalis. The use of FRP is effective because of the incorporation of special interior surface treatment, that is, the use of synthetic surfacing veils (polyesters and acrylics are common). The selection and protection Table 14.5 Temperature Limits (⬚C [⬚F]) for Various Plastics in Dual Laminate Construction in Up to 10% Caustic Soda Solutions Plastic PVC CPVC (chlorinated PVC) PE PP PVDF ECTFE (ethylene chlorotrifluoroethylene) ETFE (ethylene trifluoroethylene) FEP (fluorinated ethylene propylene) PFA (perfluoro alkoxy) Temperature Limit 40 (104), 60* (140) 80 (176) 60 (140) 100 (212) — 100 (212) 100 (212) 150 (302) 150 (302) *Conditional at this temperature, can attack or cause swelling. The medium can attack the material or cause swelling. Restrictions must be made in regard to pressure and/or temperature, taking the expected service life into account. The service life of the installation can be noticeably shortened. MS-6: Ammonia and Caustic Soda 141 of a highly resistant inner surface (the corrosion barrier) is critical for satisfactory service. The temperature limitations vary with the type of resin employed, and it should be noted that lower NaOH concentration applications may be more aggressive to FRP than the stronger alkalis because of hydrolysis effects. There are a great many resins used in conventional FRP construction. Conventional isophthalates and phenolic resins are not resistant to caustic and should not be considered. Some special formulations, such as the bisphenol-A fumarate, are very good up to 50% caustic at about 80⬚C (175⬚F). The hydrogenated Bis-A is limited to dilute caustic at room temperature. The vinyl esters are also useful in the lower concentrations of caustic soda at moderate temperatures. It must be emphasized that the chlorinated polyester (chlorendic anhydride) should not be used in NaOH. Polyurethane shows variable performance in caustic limited by the permeability of the material; the lower the permeability of the polyurethane, the better the performance. This material is limited to 10% to 15% NaOH concentration. Epoxy resin formulations are widely used up to about 90⬚C (195⬚F). Furane (furfural-furfuryl alcohol) resins can be used both as reinforced materials (e.g. with graphite) and as coatings for immersion service in intermediate concentrations. Furane is resistant to boiling 20% NaOH but not 5%. Cold-setting resins (as coatings for steel) have shown suitable resistance for up to 20% concentration NaOH even at the boiling point. Temperature limits for applicable thermoset resins in immersion service at various caustic concentrations are shown in Table 14.6 (data derived from various sources). The supplier should always be consulted in selecting thermoplastic or thermoset construction for caustic service, and adequate consideration must be given to the mechanical and physical properties, particularly thermal expansion. Carbon and Graphite Corrosion resistance of carbon and graphite in caustic is good; impervious graphite (i.e. impregnated with carbon, not organic binders) is suitable for 80% NaOH up to Table 14.6 Thermosets as FRP Maximum Temperature (⬚C [⬚F]) in Various Strengths of NaOH Resin Epoxy Furane Bisphenol-A fumarate Hydrogenated Bis-A polyester Chlorinated polyester Vinyl ester Polyurethane Note: NR ⳱ not recommended 10% 30% 50% 70% 60 (140) 60 (140) 65 (149) 25 (77) 25 (77) 60 (140) 60 (140) 60 (140) 120 (248) 65 (149) NR NR 60 (140) NR 80 (176) 120 (248) 80 (176) 120 (248) 120 NR 100 (212) NR 142 Materials Selector for Hazardous Chemicals 80⬚C (175⬚F).5 The resistance of resin-impregnated graphite depends on the resins used. Graphite impregnated with phenolic resin is marginally resistant to 10% to 15% NaOH at up to 100⬚C (212⬚F). The furane-impregnated grade is rated at up to 50% NaOH at 130⬚C, and the grade that is impregnated with fluorocarbon is claimed to be acceptable at up to 80% or even 100% at up to 230⬚C (446⬚F).6 Graphite impregnated with other resins are only slightly or not at all resistant to ⬎10% NaOH.5 Impregnated raw graphite uses modified phenolic or furane resins under high heat and pressure. The phenolic binders and cements are attacked by caustic. Impervious graphite tube-and-shell heat exchangers contain cemented joints that are traditionally made with phenolic resin for other services. It is essential that joints in equipment intended for caustic service be made with an epoxy or furane resin cements. Impervious graphite is used for seals and gaskets because it has dimensional stability combined with self-lubricating, nonfatiguing, and noncontaminating properties. Ceramics Ceramics find practically no application in caustic service. Rates of attack on glass linings are shown in Figure 14.1.7 These data show that this type of lining is suitable only for very low concentrations of caustic at very low temperatures, 1% or less at around 60⬚C (140⬚F). Figure 14.1 Wastage Rates (in mm/y) of Glass-Lined Steel in Caustic Soda. Vol: Surface Area ⳱ 20 MS-6: Ammonia and Caustic Soda 143 Rates of corrosion of borosilicate glass passes through a maximum at around 20% concentration at temperatures up to about 60⬚C (140⬚F). At higher temperatures, the maximum is at higher concentrations, and the corrosion rate at all concentrations increases dramatically. These effects are shown in the data in Table 14.7.8 The data for 50% NaOH are also presented graphically in Figure 14.2. These data Table 14.7 Corrosion Rate in mm/y of Borosilicate Glass in NaOH at Various Temperatures NaOH Concentration (%) 5 10 15 20 25 30 35 40 45 50 Figure 14.2 Temperature (⬚C [⬚F]) 41 (106) 51 (124) 65 (149) 75 (167) 100 (212) 0.007 0.010 0.012 0.012 0.010 0.009 0.008 0.007 0.006 0.006 0.015 0.021 0.024 0.024 0.020 0.017 0.014 0.012 0.011 0.010 0.055 0.063 0.071 0.071 0.071 0.067 0.063 0.055 0.051 0.051 0.126 0.154 0.178 0.190 0.197 0.190 0.166 0.150 0.142 0.137 0.87 1.18 1.42 1.58 1.74 1.85 1.90 1.90 1.82 1.78 Effect of Temperature on the Corrosion of Borosilicate Glass in 50% NaOH 144 Materials Selector for Hazardous Chemicals Table 14.8 Corrosive Weight Loss (mg/cm2/y) of Various Ceramics in 50% NaOH at 100⬚C (212⬚F) Hexoloy SA (No Free Si) 2.5 Hexoloy KT (12% Si) Tungsten Carbide (6% Co) Aluminum Oxide (99.0%) ⬎1000 5.0 75 show that glass is attacked at modest rates in 50% caustic at up to around 90⬚C (194⬚F) and above that temperature is rapidly corroded. Certain grades of silicon carbide can be used in caustic soda service. The rate of attack of silicon carbide (Hexoloy威) and other ceramics is shown in Table 14.8. The weight loss rate is given in mg/cm2/y, and a material with a rate of 0.3 to 9.9 mg/ cm2/y is considered suitable for long-term service.9 References 1. Anon, “Chemical Resistance Guide” (Bay City, MI: Dow Chemical, 1991), 20 pp. 2. Anon, “Chemical Resistance of Thermoplastics Used in Dual Laminate Constructions,” DLFA (2002), 143 pp., http://www.dual-laminate.org/html/ corrosion_guide.html. 3. P.J. Gegner, “Corrosion Resistance of Materials in Alkalies and Hypochlorites,” Process Industries Corrosion (Houston, TX: NACE International, 1975), pp. 296– 305. 4. J.K. Nelson, “Corrosion by Alkalies and Hypochlorites,” in Metals Handbook— Corrosion, vol. 13, 9th ed., ed. J.R. Davis (Metals Park, OH: ASM International, 1987), pp. 1174–1180. 5. Anon, “The Chemical Industry Build on Graphite,” brochure no. PE 200/07 (Meitingen, Germany: SGL Carbon Group, 2001), 24 pp. 6. Anon, “Corrosion Chart for Grafilor,” brochure no. GC 5 FED 7821 (Moselle, France: Le Carbone-Lorraine, undated), 22 pp. 7. Anon, “Worldwide GLASTEEL 9100,” brochure no. SB95-910-5 (Rochester, NY: Pfaudler Reactor Systems, 2000), p. 5. 8. N. Tattam, ICI PLC, unpublished report (1978). 9. Anon, “Hexoloy SA Corrosion Test in Liquids” (Niagara Falls, NY: Saint-Gobain Advanced Ceramics, 2003), http://www.carbo.com/datasheets/corrosiontest. html. 15 Corrosion in Contaminated Caustic and Mixtures There are various kinds and degrees of contamination encountered in caustic soda production and usage, and these may profoundly affect the corrosion resistance of the materials of construction commonly used in this service. As mentioned in Chapter 11, there are some impurities inherent in the manufacturing process that are substantially removed during the refining process. In other instances, the caustic soda may be mixed with other process chemicals, either deliberately or inadvertently. This chapter will discuss the effects of contamination of and by caustic and of admixtures of caustic with other chemicals. Contaminants in Caustic Soda Contaminants in caustic soda solutions derive from impurities in the feed brine, reactions occurring in the chlorine cell, or corrosion products formed during production, processing, shipping or storing. Since the source of the caustic influences the purity of the product, it should also affect the corrosiveness of the solution. A survey of producers, however, carried out by NACE found no substantial difference in the corrosion of steels or nickel alloys in either 50% or 73% caustic soda derived from either mercury or diaphragm cells. The conclusion was that differences in concentration and temperature were more important than the manufacturing method used.1 This does not mean that all caustic soda solutions are equal in terms of their corrosion behavior, and the presence of impurities does influence the attack suffered by metals and alloys. Producers go to great lengths to either reduce aggressive impurities and/or use materials that are more resistant to the type of caustic that they produce and concentrate. 145 146 Materials Selector for Hazardous Chemicals Chlorates Chlorates are produced at the anodes of the electrolytic cells and are strong oxidants. They are usually present at ⬎100 ppm and have a beneficial effect on stainless steels and an adverse effect on nickel. It has been found that nickel pickup (from corrosion in the first-effect evaporator tubing) was directly proportional to the chlorate content of the cell liquor, within the 120- to 200-ppm range encountered.2 Some plants add a continuous feed of 10% sucrose solution to react with this chlorate. No residues are left from this reaction, and product quality is not compromised.3 More commonly, the chlorate is removed before or during evaporation by proprietary treatments or by extraction with ammonia, which also reduces the dissolved salt.4 Chlorate ions can increase the rate of corrosion of carbon steel. For 48% NaOH with 0.5% NaC1O3, carbon steel showed a 10-fold increase in corrosion rate compared with caustic, which does not contain chlorate ions.5 Chlorates also increase the corrosion rate of nickel alloys as shown in Figure 15.1.4 Corrosion of nickel in the caustic plant is ascribed either to chlorate ions (ClO3) or to hypochlorites (OC1–). General corrosion rates can exceed 20 mpy (0.5 mm/y). Corrosion is aggravated by high velocity, as over welds on pump discharge piping. Velocity and increased temperature are thought to be the most probable causes of attack of nickel in caustic service rather than chlorate or hypochlorite levels. In laboratory tests at 185⬚C (365⬚F) in actual first-effect caustic liquor (43% NaOH, 0.15% C1O3) and in a synthetic mix (51% NaOH; C1O3 0.20%), corrosion resistance and stress corrosion cracking (SCC) resistance of alloy 200 and 26-1 were both superior to any of the other alloys tested. The relative resistance of the other alloys in static and circulating simulated and actual liquors was as follows: Figure 15.1 Effect of Chlorates on Various Alloys in 50% Caustic at 150⬚C (302⬚F) MS-6: Ammonia and Caustic Soda 147 alloy 600 ⬎⬎ alloy 825 艑 alloy 800 艑 alloy 28 Some of these data are summarized in Figure 15.2. This shows that chlorates increase the corrosion of nickel and nickel alloys. This accelerating effort is not sufficient to cause failures of nickel in evaporators without the presence of additional factors, such as temperature or velocity. Increased nickel content in the alloys increases the corrosion resistance, particularly in caustic without chlorates. Alloy 261, which has no nickel, has good corrosion resistance but can suffer from SCC in boiling solutions of ⬎30% NaOH and can also be subject to intergranular attack (IGA). The presence of hypochlorites was found to increase the corrosion rate of all the alloys to a lesser or greater extent.6 The effect of sodium chloride and sodium chlorate additions to hot caustic soda solutions on the corrosion of the ferritic stainless steel, E-Brite威 (S44627), is shown in Table 15.1.7 The corrosion rate of this alloy is little affected by these contaminants, common in cell liquors, unlike that of nickel, in which the corrosion rate is accelerated. This alloy is attacked in hotter, stronger caustic solutions as shown in the data for 70% at 188⬚C (370⬚F) that is included in this table.8 Corrosion is decreased in caustic by the addition of sodium chlorate and hypochlorite up to 1000 ppm, but IGA occurred when these additions were at 10,000 ppm. The effect of chorides and chlorates on the corrosion resistance of another ferritic alloy, Sea-Cure威 (S44660), in hot caustic soda solutions is shown in Table 15.2.9 These Figure 15.2 Effect of Nickel Content on Corrosion of Various Materials in Simulated First-Effect Liquor at 185⬚C (365⬚F) 148 Materials Selector for Hazardous Chemicals Table 15.1 Resistance of E-Brite威 Alloy to Caustic Solutions Containing NaCl and NaClO3 % NaOH 20 45 50 50 50 50 50 70 % NaCl % NaClO3 Temperature (⬚C [⬚F]) Corrosion Rate (mm/y [mpy]) 10 5 — 5 5 5 5 5 — — — — 0.1 0.2 0.4 0.15 104 (219) 143 (289) 143 (289) 152 (306) 152 (306) 152 (306) 152 (306) 188 (370) 0.015 (0.59) 0.041 (1.61) 0.003 (0.12) 0.076 (2.99) 0.069 (2.72) 0.028 (1.10) 0.028 (1.10) 0.110 (4.33) Table 15.2 Corrosion Rates (mm/y) of Various Alloys in Hot Caustic Solutions Alloy 316 26–1S Nickel 200 Sea-Cure 55% NaOH Ⳮ 8% NaCl Ⳮ 0.3% NaClO3 at 99⬚C (210⬚F) 50% NaOH at 143⬚C (289⬚F) 55% NaOH Ⳮ 8% NaCl Ⳮ 0.3% NaClO3 at 158⬚C (316⬚F) 0.15 ⬍0.0025 ⬍0.0025 ⬍0.0025 0.38 0.015 0.023 0.025 Very high 0.02 0.07 IGA data compare the behavior of this alloy with that of 316, another ferritic stainless steel, and pure nickel. These data show that this alloy is as resistant to these conditions as is alloy 200 but does have a tendency to IGA in this environment. The effects of the presence of chlorides and chlorates were investigated for a duplex stainless steel (S32906) and alloy 200 (N02200). These data show that when higher levels of chloride and chlorate are present, simulating membrane and diaphragm caustic, the duplex alloy is much more resistant than nickel (see Table 15.3).10 Chlorides Because NaOH is produced by electrolysis of brine, chlorides may be present from about 20 ppm to 5,000 ppm, the minimum concentration being typical of membrane cell caustic. Chlorides in caustic soda solution do not cause chloride SCC of austenitic stainless steels. A solution of 0.5 g/L NaOH at pH 12 is sufficiently alkaline to avoid this type of cracking that is common in neutral or acidic solutions.11 It has also been claimed that chlorides inhibit caustic SCC. Mercury cell caustic typically MS-6: Ammonia and Caustic Soda 149 Table 15.3 Corrosion Rates (mm/y) of S32906 and N02200 in Boiling NaOH Solutions Simulating Membrane and Diaphragm Cell Liquors S32906 N02200 50% NaOH Ⳮ 30 ppm Clⳮ, 20 ppm ClOⳮ 3 50% NaOH Ⳮ 7% NaCl, 800 ppm ClOⳮ 3 0.001 0.001 0.016 0.15 contains 20 to 30 ppm chlorides, while diaphragm cell caustic is more likely to contain up to 1% chlorides. While chloride-contaminated caustic soda solution may not cause SCC of austenitic stainless steel if the caustic soda is consumed, for example, in a neutralizing process, the residual neutral chloride containing solution may then initiate cracking.4 See also Table 15.1 for the effect of chlorides with and without chlorates on the ferritic E-Brite威 alloy (S44627), Table 15.2 for Sea-Cure威 (S44600), and Table 13.11 for the effect of chlorides on alloy 28 (N08028). Zirconium is resistant to sodium hydroxide solutions even when high levels of chlorides are present (see Table 13.16). Alloy 800 (N08800) was tested in a NaOH cooling tank at 70⬚C to 105⬚C (158⬚F– 221⬚F) for 119 days. The environment was 50% NaOH with 10% to 15% NaCl, and the corrosion rate measured was negligible at ⬍0.003 mm/y (⬍0.12 mpy).12 Chlorine/Hypochlorite Hypochlorites are formed when chlorine is introduced into water or alkaline solutions: C12 Ⳮ 2NaOH r NaC1 Ⳮ NaOC1 (1) Sodium hypochlorite (NaOC1) is liable to initiate pitting in otherwise passive metals because of the concentration cells between freely exposed and occluded surfaces, analogous to an oxygen concentration cell. The corrosion resistance of various alloys in caustic soda–containing chlorine was tested in field trials. The results of these tests showed that the chromium-nickel-iron alloy 33 (R20033) performed better than the other alloys tested (see Table 15.4).13 All the alloys showed acceptable corrosion rates in the liquid and the vapor, although pitting and crevice corrosion, severe in cases, was observed. Mercury Mercury can be entrained from the production source, while mercuric ions can be reduced to metallic mercury at local cathode sites. Metallic mercury contamination 150 Materials Selector for Hazardous Chemicals Table 15.4 Corrosion Rates (mm/y) of Various Alloys in NaOH/NaOCl Exposed to Liquid and Vapor 50% NaOH 20% NaOH Ⳮ NaOCl (80–100 g Cl2/L) 12.5% NaOH Ⳮ NaOCl (130 g Cl2/L) 110⬚C (230⬚F)— 180 Days 30⬚C (86⬚F)—195 Days 30⬚C (86⬚F)—225 Days Alloy Liquid 316 Ti ⱕ 0.01 926 654 SMO — ⱕ 0.01 C-4 — 33 ⱕ 0.01 Liquid Vapor 0.01 pitting and 0.02 pitting severe crevice corrosion ⱕ 0.01 pitting and ⱕ 0.01 severe crevice corrosion ⱕ 0.01 some — uniform corrosion ⱕ 0.01 pitting — ⱕ 0.01 Liquid Vapor — ⱕ 0.01 severe pitting ⱕ 0.01 ⱕ 0.01 some uniform corrosion ⱕ 0.01 ⱕ 0.01 ⱕ0.01 ⱕ 0.01 some crevice and uniform corrosion ⱕ 0.01 some ⱕ 0.01 some ⱕ 0.01 some crevice crevice crevice corrosion corrosion corrosion can lead to liquid metal embrittlement or liquid metal cracking in some alloy systems. Titanium and zirconium and their alloys, copper and its alloys, aluminum and its alloys, alloy 400 (N04400), and alloy 200 (N02200) at elevated temperatures are known to be at risk from this form of attack.14 Sulfur Sulfides, sulfite, or sulfate can increase the rate of corrosion of carbon steel if the passive oxide film on the steel is damaged, scratched, defective, and so on. In considering low-alloy steels for service in the cellulose and paper industry, where strongly alkaline solutions often contain sulfide, it has been found that sodium sulfide and sodium thiosulfate exacerbate damage, whereas sodium sulfite/sulfate have little effect.15 See the section “Pulp and Paper” later in this chapter. Sulfur species can also accelerate the corrosion of nickel alloys, especially at elevated temperatures. Iron Because much of the processing, storage, and transportation of caustic soda is affected in steel, iron contamination is often present, either as the soluble ferroite or MS-6: Ammonia and Caustic Soda 151 ferroate or as fines of colloidal ferric oxide. The resultant discoloration is objectionable for many applications, notably the soap/detergent, pulp/paper, and rayon/ textile industries. More resistant materials of construction are often selected just to prevent this undesirable contaminant. Sodium Hydroxide Treatments There are a number of processes that utilize caustic soda as a reactant, as a scrubbing medium, or simply as a neutralizing agent. Petroleum Refining Both sodium and potassium hydroxides are used to remove organosulfur compounds, such as mercaptans, from petroleum components in oil refineries. Economics require that the caustic must be regenerated and reused, which entails concentrations up to 45% and temperatures up to 150⬚C (300⬚F), conditions that are corrosive to steel. Stripping tower internals, tubular heaters, reboilers preheaters, and piping for handling hot caustic solutions are usually fabricated with alloy 400 (N04400). These components may be solid or clad, depending on design. Pumps and valves can be made from NiResist威 alloy cast iron.16 For operations in which aminodiisopropanol is used to remove sulfur, caustic soda is used to recover the extraction chemical. Steel would be attacked by the sulfur compounds present, but type 316L (S31603) is adequate.17 Severe caustic corrosion of the crude transfer line immediately downstream of the caustic injection point can occur when 40% caustic is injected into hot desalted crude oil to neutralize remaining HCl. This problem can be controlled by design of the injection point to ensure adequate mixing of the fluids and also is minimized if caustic is diluted to about 3% before injecting. Traces of caustic can become concentrated in boiler feedwater in boiler tubes that alternate between wet and dry conditions because of overheating. This concentrated caustic can cause corrosion, and cracking occurs under the deposits.18 Bauxite Refining In the production of aluminum from bauxite ore, alumina (Al2O3) is digested at high temperature in caustic, leaving iron oxides and silicates behind as a waste products (red mud). The aluminum oxide is then recrystallized by cooling the mother liquor. Carbon steel is used at appropriate concentrations and temperatures, with the usual provisions for stress relief. Alloy 400 (N04400) tubes are used for digester preheaters, which operate at temperatures that are too high for steel. The solution 152 Materials Selector for Hazardous Chemicals is conveyed from the heaters to the reactors in alloy 200 (N02200) or nickel-lined steel piping.17 A study aimed at finding suitable alloys to replace nickel-coated steel found that the duplex stainless steels 23% Cr, 5% Ni (S32304), and 22% Cr, 5% Ni, 3% Mo (S31803) and the austenitic alloy 28 (N08028) were much more resistant than the standard 300 series stainless steels. Any of these alloys would be suitable replacement in cases where the nickel-coated steel failed from caustic SCC.19 In a related study in typical solutions encountered in this service, 180 to 400 g/L NaOH, 10 to 20 g/L NaCl at temperatures between 180⬚C and 250⬚C (356⬚F and 482⬚F), the good resistance of duplex stainless steels was confirmed. The presence of some cracking in weld areas indicated the importance of using appropriate welding procedures. The nickel-based alloys, such as N06600 and N06625, were also resistant to general corrosion and SCC under these conditions. The 13% chromium martensitic stainless steel had insufficient resistance to general corrosion.20 In lower temperature parts of the Bayer process for alumina production, SG (spheroidal graphite) cast iron is used. Failures in this material have been identified as being due to SCC. Examination of sections that had failed in this service suggested a mechanism that involves grain boundary embrittlement ahead of SCC cracks in this material.21 Soap Manufacture Caustic is a major reactant in the saponification of fatty acids for the production of soap. Both iron and copper contamination cause rancidity in the product, and alloy 200 (N02200) is the preferred material for vessels replacing the cast iron and steel once employed. Alloy 400 (N04400) is also used and may yield a satisfactory product for some purposes despite the 30% copper component in the alloy. Corrosion rates in the top of a soap boiling kettle showed that alloy 200, alloy 400, and alloy 600 were all effectively unattacked (⬍0.1 mpy), while mild steel corroded at 3.2 mpy (0.08 mm/y) and cast iron at 11 mpy (0.28 mm/y).16 Sodium Hydrosulfide Production Sodium hydrosulfide (NaSH) is produced by the reaction of hydrogen sulfide (H2S) with 45% to 40% caustic. At reaction temperatures of about 110⬚C (230⬚F), alloy 20Cb3威 (N08020) is a suitable material of construction as compared with 316L (S31603), which is used by some producers, although it tends to suffer general corrosion. However, field corrosion tests suggest that alloy 600 (N06600) is the preferable material of construction.4 Caustic Fusion Reactions These manufacturing processes involve reaction between organic chemicals and molten caustic. In the absence of sulfur compounds, these fusions are carried out in MS-6: Ammonia and Caustic Soda 153 alloy 201 (N02201) vessels at about 315⬚C (600⬚F). Should sulfur compounds be present, as in caustic fusion of benzene metasulfonic acid to produce resorcinol (dihydroxybenzene), alloy 600 (N06600) is required because the nickel sulfide eutectic causes intergranular corrosion of nickel. Metal Finishing Molten soda ash is used in some high-temperature baths for finishing metal parts by removing metal oxides (e.g. annealing scale, mill scale). Once the reducing operation stops, there is no further reaction with the metal. Operating at about 700⬚C (1250⬚F–1325⬚F), the molten caustic acts as a carrier for 1.5% to –2% sodium hydride, a powerful reducing agent. Formed in place by reacting sodium with hydrogen, the sodium hydride (NaH) is used to descale metals and alloys by direct reduction of surface oxides. Surface oxides are removed from carbon steels, stainless steels, copper, silver, and nickel alloys by the general reaction MO Ⳮ NaH r M Ⳮ NaOH (2) and, since the reaction takes place in molten caustic, no contamination is involved. A typical treating cycle consists of the sodium hydride treatment, water quench, and water rinse, usually followed by an acid rinse and second water rinse to remove traces of caustic. The short duration of treatment effectively precludes any significant corrosion for metals and alloys that react only slowly with molten caustic.16 Solutions of sodium hydroxide are used instead of molten salts to color steel. Immersion in a caustic soda bath of 6 to 8 lb/gal at 285⬚F (140⬚C) produces a black iron magnetite coating in 10 to 20 minutes. The item is then rinsed in water, dried, and immersed in a light oil or dry-to-touch sealant to displace water and seal out atmospheric humidity to prevent rusting. This is an attractive finish with good resistance to atmospheric corrosion. The duration of treatment is too short to cause caustic cracking, which is also inhibited by oxidants present in the caustic solution.22 Pulp and Paper Pulp and paper operations encounter a wide range of caustic solutions that can cause major corrosion and cracking problems, depending on the process operated and the conditions encountered. Austenitic stainless steels are susceptible to caustic SCC above about 121⬚C (250⬚F) in pure NaOH solutions. When sulfides are present, SCC of austenitic stainless steels can occur at lower temperatures. Hot sulfide-containing caustic solution (white liquor) is used in the kraft pulping process. In different process streams of pulp mills, there are different concentrations of sulfide and hydroxide concentrations, along with other chemicals. In some areas, composite tubes with an external layer of 304L (S30403) on an inner shell of carbon steel are used, such as in the floor and lower 154 Materials Selector for Hazardous Chemicals furnace waterwalls in kraft recovery boilers. SCC occurs during boiler shutdown from exposure to the stagnant floor water that is rich in sulfides and hydroxides. A systematic study of this phenomenon found the following:23 • SCC did not occur in 304L in pure NaOH ⬍100⬚C (⬍212⬚F) in the range of solutions tested (up to saturation; ⬎30 M). • 304L stainless steels were not susceptible to SCC in Na2S solutions at temperatures up to 100⬚C (212⬚F). • In the presence of sodium sulfide in sodium hydroxide solutions, 304L stainless steel is susceptible to SCC at temperatures as low as 50⬚C (122⬚F). • Cracking susceptibility and crack velocities increased with an increase in temperature. • In recovery boilers, the solution composition possible at the floor surface can cause SCC during boiler startup at temperatures as low as 75⬚C (167⬚F). Another example of corrosion failures of stainless steels in alkaline sulfide environments in pulp mills is in the green liquor quench system. Here 316L (S31603) and 304L (S30403) stainless steels fail by corrosion in the caustic environment at 220⬚C (428⬚F) and 30 atm pressure.24 Caustic cracking of the impregnation zones of carbon-steel continuous digesters has occurred if the seam welds were not fully PWHT. Caustic solutions are used to periodically clean paper machines to remove accumulated organic matter. The solution used for this caustic boil out is at pH around 13 at 50⬚C (122⬚F). Some polymeric materials are badly attacked, high-strength steels can be corroded or subject to SCC, and copper alloys can be corroded and suffer from dealloying during this treatment.25 Caustic Contamination Caustic can be introduced accidentally into both aqueous and organic streams. Contamination of Steam A common problem in petrochemical plants is the carryover of alkaline boiler watertreating compounds in high-pressure steam. Above 300⬚C (570⬚F), the danger of caustic SCC is great. Type 321 (S32100) bellows-type piping expansion joints in 300 to 400 lb of steam are prone to rapid SCC when there is entrainment of alkaline chemicals from boiler water treatment. When high-temperature caustic SCC of stainless is encountered, there is a characteristic gunmetal blueing of the metal surface. The presence of chlorides is of no significance, as indicated by failure of alloy 800 (N08800) and 825 (N08825) replacements. Russian investigators found that chlorides do not aggravate MS-6: Ammonia and Caustic Soda 155 SCC and may, in fact, act to inhibit such attack. Alloy 600 (N06600) is satisfactory under these conditions, but alloy 625 (N06625) is currently used almost exclusively for such bellows.26 A carbon-steel steam line from the heat recovery steam generator (HRSG) to a steam host failed by caustic SCC after 6 years in service. The steam in the 24-inch (0.6-m) diameter pipe was maintained at a pressure of about 350 psi (2.4 MPa) and a temperature of 700⬚F (371⬚C). The steam temperature is reduced to about 20⬚F (11⬚C) of superheat by direct injection of boiler feedwater that contained about 40 ppb sodium hydroxide, used for pH control. The feedwater spray caused caustic soda to concentrate on the wall of a steam line elbow and produced the environment necessary for SCC. Residual stresses from the circumferential weld provided the necessary tensile stress.27 Contamination of Organic Media When caustic is inadvertently introduced into organic media, its corrosion behavior depends largely on whether the organic compound is an effective diluent. Caustic in alcohol, for example, behaves very much like aqueous sodium hydroxide. In other environments, there may be no effective dilution. A 0.2% NaOH solution in heavy glycolate tails at about 150⬚C (300⬚F) caused severe corrosion in the bottom of a steel vessel. Apparently, the dilution effect was minimal, the metal “sensing” a more concentrated caustic concentration. It was demonstrated that neutralization with concentrated sulfuric acid in stoichiometric amounts to neutralize the caustic prevented attack on steel, but there seemed no practical way to do this in a quantitative manner in plant. Replacement with a type 304 (S30400) vessel was a practical solution, providing total corrosion resistance, which seems to indicate that the effective concentration of caustic was less than perhaps 20%. Such a concentration would corrode steel but not type 304 (S30400) at the process temperature involved. Contamination of Molten Sodium Molten sodium is used as a carrier in certain high-pressure hydrogenation processes for organic compounds at 325⬚C (615⬚F). Traces of water contained in the organic compounds immediately react to form NaOH, creating an environment of small amounts of 100% caustic dissolved in molten sodium.4 There have been incidents of SCC with type 347 (S34700) heavy-walled piping when such contamination occurred. The reactor proper had been fabricated from alloy 600 (N06600)–clad steel in anticipation of possible sulfurous contaminants. Some austenitic stainless steel bolts, occasionally installed inadvertently as mechanical fasteners for the agitators, would fail by SCC within 24 hours. The original alloy 600–clad hydrogenation sphere lasted about 2 years before suffering SCC. A replacement vessel, given a thermal stress relief, lasted about twice as long. 156 Materials Selector for Hazardous Chemicals References 1. NACE Task Group T5-A report, Materials Protection and Performance 10, 7 (1971): p. 39. 2. B.M. Barkel, “Accelerated Corrosion of Nickel Tubes in Caustic Evaporation Service,” CORROSION/79, paper no. 13 (Houston TX: NACE International, 1979), 9 pp. 3. M.P. Sukumaran Nair, “Stress Corrosion Cracking—A Caustic Experience,” Chemical Engineering, January (2003): pp. 1–3. 4. C.M. Schillmoller, “Select the Right Alloys for Caustic Soda Service,” Chemical Engineering Progress May (1996): pp. 48–55. 5. K. Hauffe (1986), in Anon, NACE Network, reported in MP 42, 2 (2003): pp. 82–83. 6. J.R. Crum, W.G. Lipscomb, “Correlation between Laboratory Tests and Field Experience for Nickel 200 and 26–1 Stainless Steel in Caustic Service,” CORROSION/83, paper no. 23 (Houston, TX: NACE International, 1983), 18 pp. 7. Anon, “E-Brite Alloy,” technical data sheet no. B-150-Ed1-10M-181P (Pittsburgh, PA: Allegheny Ludlum Corp., 1980), p. 7. 8. J.R. Kearns, M.J. Johnson, I.A. Franson, “The Corrosion of Stainless Steels and Nickel Alloys in Caustic Solutions,” CORROSION/84, paper no. 146 (Houston, TX: NACE International, 1984), 18 pp. 9. Anon, “Trent SEA-CURE Stainless Steel for Power Generation and Chemical Processing,” brochure no. A18-7/00-5000 (East Troy, WI: Trent Tube, 2000), 20 pp. 10. D. Leander, “Corrosion Characteristics of Different Stainless Steels, Austenitic and Duplex, in NaOH Environment,” Stainless Steel World Conference, Maastricht, Netherlands (2003), 9 pp. 11. B.D. Craig, D.B. Anderson, eds., Handbook of Corrosion Data (Materials Park, OH: ASM International, 1997), pp. 761–790. 12. Anon, “Incoloy Alloys 800, 800H and 800HT,” brochure no. 1A1 172/7M (Huntington, WV: Inco Alloys International, 1997), 28 pp. 13. M. Kohler, U. Heubner, K.W. Eichenhofer, M. Renner, “Progress with Alloy 33 (UNS R20033), a New Corrosion Resistant Chromium Based Austenitic Material,” CORROSION/96, paper no. 428 (Houston, TX: NACE International, 1996), 18 pp. 14. J.R. Davis, ed., Corrosion—Understanding the Basics (Materials Park, OH: ASM International, 2000), p. 191. 15. D.A. Wensley, R.S. Charlton (1980), in “Sodium Hydroxide Advisor,” ChemCor 6 (MTI/NACE/ NiDI/NIST, 1992). 16. Anon, “Corrosion Resistance of Nickel and Nickel-Containing Alloys in Caustic Soda and Other Alkalies,” CEB-2 (New York, NY: International Nickel Company Inc., 1973), 40 pp. 17. C.M. Schillmoller, “Alloy Selection for Caustic Soda Service,” NiDI technical series no. 10019 (Toronto, ON, Canada: NiDI, March 1988), 9 pp. 18. J. Gutzeit, R.E. Merrick, L.R. Scharfstein, “Corrosion in Petroleum Refining and Petrochemical Operations,” in Metals Handbook—Corrosion, vol. 13, 9th ed., ed. J.R. Davis (Metals Park, OH: ASM International, 1987), p. 1269. MS-6: Ammonia and Caustic Soda 157 19. A. Cigada, G. Rondelli, B. Vincentini, M.F. Brunella, “Caustic Stress Corrosion Behavior of Some Duplex and Austenitic Stainless Steels,” Proc. 3rd IberoAmerican Congress of Corrosion and Protection, Brazilian Corrosion Congress, vol. 1 (1989), pp. 223–233. 20. A. Cigada, M.F. Brunella, M. Cabrini, G. Rondelli, B. Vincentini, S. Ventura, “Stress Corrosion Behaviour of Duplex Stainless Steels in Caustic Environments: Laboratory and Field Experiences,” Proc. 14th ICC, paper no. 32.0 (1999), 7 pp. 21. R.K. Singh Raman, B.C. Muddle, R.M. Tomlins, “Stress Corrosion Cracking of Steels and Their Weldments in Strong Caustic Environments,” paper no. 38-065, Corrosion and Prevention 1998 Conference (1998), 7 pp. 22. M. Ruhland, “In-House Blackening of Ferrous Metals” (Eden Prairie, MN: Birchwood Laboratories Inc., 2003), http://www.birchwoodcasey.com/presto/ presto-9359.html. 23. P.M. Singh, O. Ige, J. Mahmood, “Stress Corrosion Cracking of 304L Stainless Steel in Sodium Sulfide-Containing Caustic Solutions,” CORROSION/2003, paper no. 03518 (Houston, TX: NACE International), 14 pp. 24. J.R. Keiser, R.A. Peascoe, C.R. Hubbard, J.P. Gorog, “Corrosion Issues in Black Liquor Gasifiers,” CORROSION/2003, paper no. 03354 (Houston, TX: NACE International), 19 pp. 25. A.H. Tuthill, ed., “Stainless Steels and Specialty Alloys for Modern Pulp and Paper Mills” NiDI reference book no. 11 025 (Toronto, ON, Canada: NiDI, 2002), 152 pp. 26. C.P. Dillon, “Corrosion of Stainless Steel by Hot Caustic,” MP 37, 1 (1998): pp. 64–65. 27. F.C. Anderson, P.S. Jackson, D.S. Moelling, F.M. Glasgow, “HRSG Tube Failures: Prediction, Diagnosis and Corrective Actions,” CORROSION/2003, paper no. 03495 (Houston, TX: NACE International), 12 pp. 16 Related Chemicals There are a number of chemicals related to caustic soda that are really outside the scope of this monograph but some of which should at least be introduced here for completeness. The first is chlorine, whose production is intimately tied to caustic soda production. Materials of construction for chlorine have, however, been fully described in another MTI monograph1 in this series and will not be discussed here. Soda Ash Soda ash is anhydrous sodium carbonate (Na2CO3), CAS number 497-19-8. It exists as a naturally occurring mineral in parts of Africa and in the United States, notably in Wyoming. Native soda ash is found in the form of Trona, which is sodium carbonate and bicarbonate, or as brines that are mixtures of sodium carbonate, sulfate, and sulfite.2 Soda ash can be produced by the reaction of caustic soda and carbon dioxide or in the Solvay (ammonia soda) process that reacts limestone, ammoniated brine, and coke. Soda ash is used in household cleaners (washing soda), glass making, water treatment, chemical processing, and so on. In some pulp and paper applications, soda ash is reacted with limestone to produce caustic soda, used in neutralizing and other processes. Pure soda ash is a hygroscopic white powder whose molecular weight is 105.99 and specific gravity 2.533. It has a melting point of 851C (1564F) and generates heat of solution in water. It has a strong degree of alkalinity. The sodium carbonate decahydrate (Na2CO3 •10 H2O) melts at 34C (93F) to form a solution of about 37% concentration. Corrosion problems may develop because it can either lose carbon dioxide to release free caustic or further absorb CO2 to form the bicarbonate (NaHCO3). Sodium carbonate at elevated temperature will cause caustic corrosion in a manner similar to caustic soda corrosion. The propensity to caustic stress corrosion cracking (SCC) is less with soda ash, and carbon steel is routinely used to handle boiling 159 160 Materials Selector for Hazardous Chemicals solutions. The alkalinity of soda ash is less than that of NaOH, and the carbonate ions have an inhibiting effect on many metals. Soda ash can form the bicarbonate that may corrode steel because of its lower pH.3 A typical application is the use of sodium carbonate solutions for the absorption and release of carbon dioxide from hydrocarbon streams, in a manner analogous to alkanolamine acid gas scrubbing systems. The carbonate reacts selectively with CO2 to form the bicarbonate, which is reheated in the separation column to release the acid gas, returning the regenerated lean Na2CO3 solution to the absorber. This process is inherently corrosive to steel, particularly in the regeneration step. Addition of oxidizing inhibitors such as sodium vanadate is used to control corrosion in the stripping still, and type 304 (S30400) reboilers are employed to resist corrosion in the reboiler. Potassium Hydroxide Potassium hydroxide (KOH), CAS number 1310-58-3, is very similar to sodium hydroxide in its chemical and corrosion characteristics, but it is much less common. Caustic potash is produced in a similar manner to caustic soda by the electrolysis of potassium chloride. The equipment for evaporation is based largely on nickel and nickel alloys with cathodic protection being employed for 50% KOH solutions, which have a higher boiling point than NaOH. Corrosion data is limited, but KOH corrosion behavior is similar to that of NaOH, so we can usually extrapolate from the NaOH data and experience. Caustic SCC can occur in caustic potash solutions at elevated temperatures, but cracking is not so severe in the potassium salt for alloy 400 (N04400) and alloy 600 (N06600). A failure of a low-carbon pressure vessel after 10 years of service in a hydrogen sulfide absorber was diagnosed as due to caustic SCC from KOH. The environment was 20% aqueous KOH, potassium carbonate, and arsenic at 33C (91F). Although the operating temperature is low for SCC, the vessel had been exposed to a fire that may have taken the temperature above the induction temperature. It may also have increased the local concentration of KOH and added stress to the existing residual and operating stresses.4 In a problem analogous to that which occurs with caustic carryover in steam, SCC of type 347 (S34700) by potassium hydroxide has been reported. In one plant, a catalyst containing potassium oxide was to be used in a hydrogenator. When the plant was started up with 300C (570F) steam after a catalyst change, sufficient KOH was formed and entrained to cause failure of the stainless equipment downstream. Some data are available for alloy 200 in caustic potash (KOH) under velocity conditions and when mixed with potassium chloride (KCl) and potassium chlorate (KClO3) (see Table 16.1).5 Data for various metals and alloys in KOH are shown in Table 16.2.6 This table shows that KOH at these temperatures and concentrations is not very corrosive even to low-carbon steel. MS-6: Ammonia and Caustic Soda 161 Table 16.1 Laboratory Corrosion Tests in Potassium Hydroxide Environment 30% NaOH, saturated, with KCl Ⳮ 0.05% KClO3 47% NaOH saturated, with KCl Ⳮ 0.078% KClO3 Liquid Vapor Liquid Vapor 50% KOH 6.6 m/min 106 m/min 6.6 m/min 106 m/min 70% KOH Temperature (C [F]) Corrosion Rate (mm/y [mpy]) Boiling 0.005 (0.20) 0.008 (0.31) 0.002 (0.08) 0.008 (0.031) Boiling 150 (302) 150 (302) Weight gain Weight gain 0.01 (0.39) 0.04 (1.57) Table 16.2 Corrosion Rate (mm/y [mpy]) of Metals and Alloys in KOH Solutions Alloy 13% KOH at 30C (85F) 13% KCl added 50% KOH at 25C (80F) 0.023 (0.9) 0.005 (0.2) Nil Nil Nil 0.013 (0.5)* 0.01 (0.4) 0.0015 (0.06) 0.00008 (0.003) 0.00005 (0.002) Nil 0.0013 (0.05) Titanium Zirconium Nickel Monel Inconel Low C steel *Slight attack under spacer Zirconium has a corrosion rate of 0.025 mm/y (1 mpy) in 0% to 50% KOH at temperatures up to boiling.7 Zirconium in 50% KOH at 27C (81F) corroded 0.06 mpy (0.0015 mm/y), and in short-term tests in 50% to anhydrous KOH at 241C to 377C ( 466F–711F), the corrosion rate was 0.03 mpy (0.001 mm/y).8 References 1. C.P. Dillon, W.I. Pollock, eds., Materials Selector for Hazardous Chemicals: Hydrochloric Acid, Hydrogen Chloride and Chlorine, vol. MS-3 (St. Louis, MO: MTI, 1995), 200 pp. 2. Anon, “Lake Natron Soda Ash Project” (Dar es Salaam, Tanzania: National Development Corp., 1997), http://www.ndctz.com/sodaash.htm. 3. J.K. Nelson, “Corrosion by Alkalies and Hypochlorites,” in Metals Handbook— Corrosion, vol. 13, 9th ed., ed. J.R. Davis (Metals Park, OH: ASM International, 1987), pp. 1174–1180. 162 Materials Selector for Hazardous Chemicals 4. G.W. Powell, S.A. Mahmoud, eds., Metals Handbbook—Failure Analysis and Prevention, vol. 11 (Metals Park, OH: ASM International, 1986), p. 658. 5. Anon, “Wiggin Corrosion Resisting Alloys” (Hereford, UK: Wiggin Alloys Ltd, 1983), p. 18. 6. P.J. Gegner, W.L. Wilson (1959), in J.K. Nelson, “Corrosion by Alkalies and Hypochlorites,” in The Metals Handbook—Corrosion, vol. 13, 9th ed., ed. J.R. Davis (Metals Park, OH: ASM International, 1987), pp. 1174–1180. 7. K. Bird, “The Caustic Truth about Zircadyne威,” Outlook 15, 3 (1994): pp. 6–7. 8. D.R. Knittel, R.T. Webster, “Corrosion Resistance of Zirconium and Zirconium Alloys in Inorganic Acids and Alkalies,” ASTM Symposium on Industrial Applications of Zirconium and Titanium (1979). 17 Summary of Corrosion of Materials in Caustic Soda The detailed behavior of materials in caustic soda has been discussed so far. In summary, mild steel is acceptable for most applications of low-temperature, lowconcentration caustic soda except where the avoidance of iron contamination is critical. The 300 stainless steels can be used at higher temperatures and concentrations or to reduce iron contamination. At higher concentrations and temperatures, more highly alloyed materials are used, including nickel-based alloys and austenitic, ferritic, and duplex stainless steels. For the hottest, strongest solutions, nickel is preferred unless oxygen or oxidizing agents are present. In that case, super stainless steels of nickel alloys are specified. The two factors that mainly control the limits of applications of materials are resistance to metal loss (general corrosion) and resistance to caustic stress corrosion cracking. These factors are summarized for common alloys in Figures 17.1 and 17.2. The curves for stainless steels (304/316) and nickel in Figure 17.1 and for nickel-rich austenitic alloys in Figure 17.2 are speculative and not based on rigorous statistical testing. These curves are included with the “standard” curves as a suggested comparison. The range of applications for various nickel alloys is shown in Figure 17.3. Above 320⬚C (608⬚F), alloy 201 (N02201) should be used. There have been attempts to summarize applicable limits of concentration and temperature. One such summary is shown in Table 17.1.1 This is a useful summary given the proviso that the actual behavior of any of these alloys does depend on other factors such as velocity, the presence or absence of contaminants, and so on. Selection of a materials also depends on the application. For example, the material recommended for a heat exchanger cooling recirculating caustic might be different from the material used in a once-through pipeline handling the same strength and temperature of solution. Reference 1. Anon, “Alloys for Caustic Soda Service by Corrosion Resistance Category” (Houston, TX: The Hendrix Group Inc., 2003), http://www.hghouston.com/ naoh.html. 163 164 Figure 17.1 Figure 17.2 Materials Selector for Hazardous Chemicals Isocorrosion Curve (at 1 mpy) for Stainless Steel and Nickel in Caustic Soda Summary Curves for SCC Regions for Carbon Steel, Stainless Steel, Nickel-Rich, and Nickel-Based Alloys MS-6: Ammonia and Caustic Soda Figure 17.3 165 Range of Use of Various Nickel Alloys in Caustic Soda Table 17.1 Alloys for Caustic Soda Service by Corrosion Resistance Category Category A alloys are useful at all concentrations and temperatures. Alloy 200 (N02200) Category B alloys are useful up to 350⬚F (176⬚C) and 50% concentration. May show useful resistance up to 600⬚F (315⬚C) but should be tested first. Alloy 690 (N06690) Alloy 600 (N06600) Alloy 400 (N04400) Alloy Ebrite 26–1威 (S44627) tubing and piping only Category C alloys are useful up to 50% caustic at atmospheric boiling (300⬚F [149⬚C]). May exhibit similar resistance to category B alloys but based on limited data should be tested first. Alloy 625 (N06625) Alloy C-276 (N10276) Alloy B-2 (N106650) Category D alloys are useful up to 250⬚F (121⬚C) and 50% concentration. Alloy 800 (N08800) Alloy 20 (N08020) Category E alloys are useful up to 200⬚F (93⬚C) and 50% concentration. Type 304 (S30400) Type 316SS (S31600) 18 Specific Production Equipment This chapter covers details relating to specific items of equipment as well as recommendations for such equipment as determined by environment and service conditions. It provides materials recommendations for specific equipment in sodium hydroxide service. This chapter also covers aspects of design and operation that are relevant to particular types of equipment. Production plant equipment includes reactors, heat exchangers, condensers, liquefiers, evaporators, dryers, fractionating stills/columns, boilers, heaters, and crystallizers. A corrosion engineer should be consulted when contamination is a potential problem or where the caustic is mixed with other chemicals. Production Equipment Caustic soda is normally coproduced with chlorine using a variety of different systems. The materials for the construction of mercury, diaphragm, or membrane chlorine cells are discussed in the MTI Monograph 3. This monograph also describes the process and materials used to prepare the brine prior to hydrolysis, and this stage of caustic production will not be dealt with here.1 The weak, impure caustic soda solution from the diaphragm cell is treated to remove some of the impurities and then concentrated using multiple-effect evaporators. A summary of some of the areas in caustic production in which nickel alloys are being successfully used is shown in Table 18.1.2 Other alloys and materials are used for some of these items of equipment, and these will be discussed here. Pressure Vessels Pressure vessels must be designed and fabricated to meet the provisions of Section VIII of the ASME Code. Carbon steel is used within the limiting parameters of 167 168 Materials Selector for Hazardous Chemicals Table 18.1 Nickel Alloys Used in Caustic Soda Production Equipment Brine pumps Brine heaters Evaporator Material Bodies Steam chests Anhydrous Heat exchangers Pumps Bodies Shafts Impellers Valves and fittings Pipelines Filters Bodies and drums Filter cloth, backing, and winding wire Piping, valves, and fittings Settling tanks Crystallizers Bodies Shafts and agitators Centrifuges Tanks cars Bodies Coils Transfer piping Ni-Resist威, alloy 400 Alloy 200, alloy 400 Ni-clad or alloy 200-sheet lined steel Alloy 200 or alloy 400 tube sheets Alloy 200 or alloy 400 or Ni-clad downtakes Alloy 200 or alloy 400 tubes Alloy 201 or alloy 600 tubes Alloy 200 or alloy 400 Alloy 200 or alloy 400 or Ni-Resist威 Alloy 200 or alloy 400 Alloy 200 or alloy 400 or Ni-Resist威 Alloy 200 or alloy 400 or Ni-Resist威 Alloy 200 or alloy 400 or Ni-Resist威 Alloy 200 or Ni-clad steel Alloy 200 or alloy 400 Alloy 200 or alloy 400 or Ni-Resist威 Ni-clad steel or lined with alloy 200 sheet Ni-clad steel or lined with alloy 200 sheet Alloy 200 or alloy 400 Alloy 400 baskets and wire cloth liners Ni-clad steel Alloy 200 Alloy 200 or alloy 400 concentration and temperature, with nickel, nickel alloys, and stainless steels being used at higher NaOH concentrations and temperatures. For carbon steel, the ASME Boiler and Pressure Vessel Code allows stress relief at temperatures as low as 482⬚C (900⬚F). However, this is not reliable for caustic soda service. A typical stress relief for a carbon-steel vessel in caustic service would be to hold at 593⬚C (1100⬚F) for 1 hour per inch thickness, with a minimum of half an hour. There are no fabrication requirements for stainless steel that specifically relate to caustic service. Stainless steel vessels need not be stress relieved because chloride stress corrosion cracking (SCC) does not occur in caustic service. Nickel and its alloys (although ductile and malleable in the annealed condition and readily fabricated by normal techniques) require stress relief for some applications. Highly stressed components should be stress relieved at 480⬚C to 870⬚C (900⬚F–1600⬚F). Alloy 400 (N04400) can experience embrittlement in the range 650⬚C to 870⬚C (1200⬚F–1600⬚F), so the alloy is normally hot worked at about 1040⬚C (1900⬚F). MS-6: Ammonia and Caustic Soda 169 Recommendations for effective stress relief are important especially in hot concentrated solutions of caustic where SCC is possible even for some of the stronger nickel-bases alloys, such as alloy 625 (N06625). All high-nickel alloys are extremely sensitive to even traces of sulfur compounds that are absorbed by the metal at the welding temperature, causing serious embrittlement. Welding must be undertaken with extremely clean conditions. Brine Circulation Piping Brine circulation piping has been made from various grades of stainless steels, nickel, or nickel alloys or NiResist威 cast iron. FRP and dual laminate piping is also being successfully used in this application, as is rubber-lined carbon steel. Evaporators and Crystallizers The primary concern in caustic production is the multiple-effect evaporator. In the past, caustic evaporators and crystallizers were made from gray cast iron, but now they are usually made of nickel alloy 200 (N02200) in the form of steam-heated tubular exchangers. Alloy 201 (N02201) is specified when operating above 315⬚C (600⬚F). Solid nickel, nickel clad, or nickel lined have all been used in this application. For production of concentrations above 73%, the heating medium is a hightemperature heat transfer fluid or a molten salt. For this duty, operating at temperatures above 315⬚C (600⬚F), alloy 201 (N02201) is used since alloy 200 (N02200) can be subject to graphite formation and embrittlement. In some cases, stressrelieved alloy 600 (N06600) has been used instead of alloy 200 (N02200), particularly when sulfur compounds are known or suspected to be present in the liquor to be concentrated.3 Where chlorate contamination is a problem (causing accelerated corrosion of nickel), some operators have used the ELI 26-1 (S44626), depending on the chlorates, to maintain passivity. The ferritic alloy E-Brite威 has often been used successfully, but there have been some failures. Failures of 26-1 ferritic alloys in this service have been due to intergranular attack (IGA). Short-term, low-temperature testing does not cause IGA, but such attack becomes more probable as time and temperature increase. Contributory factors include crevices, corrosion products, and perhaps elemental sulfur. This type of alloy is also susceptible to SCC in hot caustic solutions at ⬎30% concentration. The most probable cause of alloy 200 failures in caustic evaporators is increased temperature and velocity.4 Another superferritic stainless steel, alloy 29-4 (S44700), has excellent resistance to boiling 50% NaOH, even in the presence of chlorates, and has become a standard material for caustic evaporator steam chests and other associated heat exchangers.5 While most evaporators are based on a shell and tube design, plate exchangers are also now being used to concentrate 32% membrane caustic up to 50%.6 The presence of sodium hypochlorite in diaphragm cell liquor has caused corrosion of nickel and 26-1 in first-effect evaporators. Sodium chlorate in the cell liquor 170 Materials Selector for Hazardous Chemicals has caused accelerated attack of nickel. In the presence of chlorates, the ferritic steels are better than nickel since they tend to be passivated by this oxidizing chemical. In some cases, the cell liquor is treated to reduce corrosion in the evaporators. For example, chlorates are often removed by ammonia extraction, proprietary treatments, or additions of sucrose. Sodium sulfite is added to reduce hypochlorite.7 Other inhibitor chemicals are added to reduce corrosion rate. One of these is a reducing chemical, sodium borohydride, said to reduce corrosion due to high temperatures and turbulence. Treatment with this chemical can reduce nickel pickup across evaporators by up to 50% to 80%. This same treatment also reduces other metallic contaminants, such as iron.8 To produce solid caustic soda, one method is to use a falling film evaporator that provides heat and gradually removes water from the original 50% NaOH solution. One such evaporator is made from alloy 201 tubes and is heated with molten heat transfer salt. This unit failed after 18 months of service by a combination of pitting and longitudinal cracking, mainly in the bottom third of this vertical heater. The failure was diagnosed as being caused by a combination of intergranular SCC and accelerated corrosion due to local overheating. The excessive stress derived from conditions during shutdown and startup. Improved operating conditions to avoid dry, hot spots and control of chlorates have been instituted with the replacement unit. Once the fused caustic mass leaves the evaporator, it is broken up in a flaker drum. In this particular plant, this unit also failed prematurely by cracking that was diagnosed as being due to residual fabrication stress and operating thermal stresses. Since carbon precipitation may have been a factor in the failure of this alloy 200 (N02200) flaker drum, the replacement will be made from alloy 201 (N02201).9 Salt Separators Salt settlers are usually fabricated from nickel alloy 200 (N02200) to resist chloride concentration cell effects, while slurry tanks are usually alloy 400 (N04400). Salt settlers often incorporate cooling using alloy 200 for the heat transfer surfaces. A failure of one of these nickel heat exchangers was caused when seawater was used as the coolant. It was replaced with alloy 400 (N04400) welded with alloy 625 (N06625).2 Austenitic stainless steels have been used in salt recovery systems and have failed from SCC. Caustic Soda Handling All manufacturers and transporters of solid caustic soda or caustic soda solutions provide information regarding safe handling and equipment needed to deal with these products. This information is available in the form of MSDS (Materials Safety Data Sheets) and in specific brochures available from the suppliers. There is also a MS-6: Ammonia and Caustic Soda 171 comprehensive document prepared by the Chlorine Institute that gives guidance and detailed information on the handling of caustic solutions.10 Some of the principal features of materials and equipment suitable for handling caustic are presented in this section. Heat Exchangers Heat exchangers of various types and designs are used to add heat, such as to prevent freezing, or to remove heat, such as to make caustic soda solutions cool enough for storage. Heaters Heaters are needed in storage tanks to maintain caustic solutions liquid. Internal coils, bayonets, or U-tubes are made from alloy 200 (N02200) or alloy 400 (N04400). Alternatively, external shell and tube heat exchangers can be used to provide the necessary heat to prevent freezing. These are normally made with nickel tubes and steam heating on the shell side. These external heaters have the advantage of ease of inspection and maintenance. Coolers Heat exchangers used for cooling must be resistant not only to caustic but also to the cooling water being used. For example, a 6% molybdenum austenitic stainless steel might be used for a high-chloride water if the process-side conditions of caustic concentration and temperature permit. More often, alloy 400 (N04400) or alloy 625 (N06625) is chosen for brackish or seawater cooling. However, in one caustic membrane heater/cooler handling 33% NaOH, failures were observed as coming from the seawater inside the tube, probably because of marine fouling, microbial corrosion, or the alternating exposure to steam and seawater. Plate heat exchangers are more often used than shell and tube exchangers for removing heat from caustic dilution operations. Type 304L (S30403) or 316L (S31603) are used for low temperatures and concentrations, with alloy 400 or nickel used under more aggressive conditions (⬎60⬚C [⬎140⬚F]). Elastomeric gaskets have been successfully employed with safety screens to avoid caustic spray in case of a gasket failure. Storage Tanks The basis of the design for metallic, vertical, cylindrical storage tanks for caustic solutions can be API 12F,11 API 620,12 or API 65013 standard, depending on the design parameters. An appropriate design standard for metallic, horizontal tanks is ASME Section VIII,14 while for FRP tanks, ASME RTP-115 is appropriate. Bare carbon steel is commonly selected for storage of ⬍51% NaOH at up to 50⬚C (122⬚F) unless iron contamination is a problem. Such vessels should be stress relieved if operating within the parameters defined by Figure 13.4. Carbon steel does not need to be stress relieved when it is coated or when the vessel is operated 172 Materials Selector for Hazardous Chemicals consistently outside the ranges in which stress relief is required. Some operators, however, choose to stress relieve carbon-steel tanks even when operating in the “safe” region. At higher concentrations and temperatures, accelerated corrosion and SCC can occur, and other materials should be selected. The standard low-carbon 300 series stainless steels have been used successfully, but they must be protected from external chloride SCC. Ferritic or duplex stainless have also been used because of their superior resistance to chloride SCC. Nickel and nickel alloys are satisfactory for any combination of temperature and concentration and are normally used in high-concentration or critical purity applications. For storage of 73% NaOH, nickel or nickel clad on carbon steel is commonly used. FRP is not common for caustic storage but may be used if metal contamination must be avoided. FRP tanks need structural strength, must be able to be heat traced, and have a resistant resin (e.g. vinyl or epoxy) and an appropriate, internal corrosion barrier. Linings such as PVC, CPVC, PP, polysulfone, or fluorocarbons are used in ⬍50% NaOH, usually in the form of dual laminates with FRP structural. Other lining materials that are used in appropriate applications include epoxy, vinyl, natural rubber, neoprene, and chlorobutyl rubber. Regardless of the material used for the tank, care must be taken over all aspects of fabrication. Errors, shortcuts, mix-ups, and general lack of supervision or clear instruction to fabricators and/inspectors and so on account for many unnecessary failures in process plant and equipment. Residual tensile stresses resulting from cold forming, such as cutting, bending, and twisting, or from welding can cause SCC unless stress relief is subsequently applied. Welding always produces localized stresses above the yield point because of restraint under cooling and may be vulnerable to SCC at the operating concentrations and temperatures. A postweld beat treatment (PWHT) for stress relief effectively reduces the stresses to below the critical threshold stress level, where caustic embrittlement will not occur. Carbon steel should be stress relieved at a minimum of 593⬚C (1100⬚F), holding the material at temperature for 1 hour per inch of thickness for at least 0.5 hours. Stress relief, typically in the range 550⬚C to 650⬚C (1020⬚F–1200⬚F), increases the safe working temperature by at least 20⬚C (36⬚F). It must be noted, however, that on-site procedures may not permit fully effective stress relief. Compromise approaches require full attention to temperature distribution and control, times and cooling, and so on. Methods include the following: • Sectional stress relief, using a furnace on horizontal sections of fabricated equipment • Field insulation with heat input to total vessel, which demands very careful control and inspection • Local section stress relief, using portable heating tapes or resistance heaters • Welding torch stress relief, where unevenness of temperature, temperature control, times, and cooling rates are more in question than expected for the other techniques Furthermore, if operating stresses are high, they can cause SCC, the time to cracking diminishing as the magnitude of the stresses increase. MS-6: Ammonia and Caustic Soda 173 Fabricated carbon steel, used for handling and storing NaOH at up to 50% concentration, does not generally require thermal stress relief because the storage temperature is usually less than 60⬚C (140⬚F). Overheating can still occur because of direct exposure to the sun or if there is a loss of control of the heat applied to prevent freezing. Under the influence of this localized heating, an apparently nonaggressive caustic solution can become considerably more corrosive and cause SCC in susceptible materials. There have been cases of caustic embrittlement (SCC) when soil subsidence placed stress-relieved storage tanks in a bending moment, reintroducing tensile stresses. In the design of both storage tanks and other process (“day”) tanks or vessels, certain detailed service requirements must be considered. These include the following: • The expected caustic concentration and possible concentration changes. • Possible changes in the quality of caustic from various sources and the type and extent of impurities. • Exposure to air, condensation, and dilution effects as well as the potential for evaporation or concentration. • The operating temperature and the appropriate heating method to avoid freezing. • Tank holding (residence) time; frequency of use and duration of exposure of volume per unit area are related to the tolerance of caustic regarding iron contamination. • It should be noted that high local concentrations of caustic can occur in certain undrained areas of a tank, in horizontal feed nozzles, or from localized heating. Residues of caustic can remain in a vessel that has to be repaired after prior service. Localized concentration effects can occur because of local overheating and when residual stresses are reimposed, which could cause unexpected SCC. Thorough cleaning prior to repair work is essential. Stressed areas of carbon steel, exposed to hot strong caustic, rarely suffer from SCC after a lining ultimately fails. It has been suggested that the steel effectively undergoes a low-temperature stress-relieving period during the life of the coating.16 Tanks require routine cleaning because sodium carbonate precipitates out of stored caustic soda, forming a hard crust on the tank floor. Removing the precipitated carbonate is important to maintain product quality and to perform tank inspections. Large caustic storage tanks (250,000 gallons and more) are typically cleaned and/or inspected every 10 years, while smaller tanks are often cleaned on a more frequent basis. Carbonate buildup can be reduced by correct tank design and operating procedures.17 Piping The most common and economically justified material for piping is carbon steel usually designed and fabricated to ANSI/ASME specification B31.3.18 Alternative 174 Materials Selector for Hazardous Chemicals materials, both metallic and nonmetallic, are needed to comply with piping code restrictions (conditions beyond the physical limits of carbon steel) or where contamination is a problem. Caustic SCC of steel occurs above threshold temperatures when stresses exceed the yield point. Steel pipe needs to be stress relieved not only when welded but also when cold formed, that is, when bent, field flared, and so on. The stainless steel types 304L (S30403) and 316L (S31603) have been used in caustic piping systems; the low-carbon grade gives improved corrosion resistance in the weld zone. In applications where the austenitic steels may suffer from SCC, alloy 400 (N04400) has sometimes been used, but for high-temperature, highconcentration applications, nickel is usually specified. There are many factors that influence the behavior of piping systems and the selection of suitable materials. Contributing factors include the following: • Pressure/temperature limitations—especially for nonmetallic materials of construction. • Support structures—nonmetallic systems will require additional support. • Tracing/insulation requirements—direct contact of steam tracing with the pipe must be avoided. Steam tracing should match the pumping schedule for the caustic; overheating can result when static conditions obtain. Polypropylene- and PTFE-lined steel pipe have both been utilized as alternatives to steel to circumvent the overheating problem. • Corrosion resistance of material(s), including concentration effects and impurities and flow effects (bends, joints, valves, and so on)—within normal design parameters, velocity is not a major factor. • Fabrication—permanent joints or dismountable, gaskets and sealants to be compatible with caustic, and welds (residual stresses, compatibility, and so on). • Physical effects, such as thermal expansion/contraction. Special safety measures are often required for piping systems handling hazardous chemicals. Safeguards that are described in ANSI/ASME B31.3 specification include the following: • Plant layout to provide isolation or controlled access • Protective barricades or other systems for collecting or recovering spillage • Operating practice, including work permits, special training, and similar provisions • Engineering design features, such as insulation, shock protection, armoring, and double containment This specification pertains only to piping and equipment and advises on safeguarding by armoring or other means. However, special consideration should also be given to the following: • Proper support • Provision for thermal expansion • Protection against mechanical damage, such as by water hammer MS-6: Ammonia and Caustic Soda 175 For commercial strengths of 50% or more, heat tracing is routine to prevent freezing. This can introduce problems because service experience shows that heat tracing can produce a local increase in temperature (and/or increase in caustic concentration) beyond the values of the bulk fluid. These effects need to be taken into account when optimizing candidate material(s). Self-regulating electric heating tape is recommended. Other heating tapes or cables with thermostatic control may also be used. Steam tracing is not normally recommended since the temperature of caustic soda can readily exceed 140⬚F (60⬚C) under static conditions, causing eventual SCC. Insulation and weatherproofing are also required if the piping must be heated. If maintaining low iron concentration in the caustic soda solution is important, use a flanged steel pipe with a polypropylene lining. Unsupported plastic pipe should not be used for caustic soda, and fiberglassreinforced plastic pipe should be used cautiously only for specific applications.19 Pumps Pumps for transferring caustic from storage to point of use are typically centrifugal, although positive displacement or other types are used for specific duties. Magnetically driven, sealless pumps are often recommended for caustic service. Cast steel or ductile cast iron pumps may be used for up to 50% caustic to about 65⬚C (150⬚F), provided that iron contamination is not of concern. Bronze pumps, sometimes with stainless steel impellers, may be used for intermediate strengths of caustic if copper contamination is not objectionable. To avoid such contamination, the cast austenitic stainless steels, such as CF8M (J92900), are the most convenient commercially available materials. From 65⬚C (150⬚F) to 100⬚C (212⬚F), electroless nickel plating (ENP) may be used simply to minimize iron contamination, or cast alloy 20, such as CN7M (N08007), may be used. A cast duplex stainless steel such as CD4MCu (J93370) is also used. However, cast nickel alloys are to be preferred, such as alloys CZ100 (equivalent N02200), M35 (equivalent N04400), and CY40 (equivalent N06600). Above 100⬚C (212⬚F), only the nickel alloys or fluorocarbons should be specified. Aluminum, magnesium, copper, brass, zinc, and tantalum must all be avoided in caustic pump construction. Heat tracing will need to be provided if the pump operates at ⬍60⬚F (⬍15.6⬚C) in 50% caustic solutions. Steel or cast iron pumps used for handling ambient 50% caustic solutions can need frequent maintenance and have short lives. A more durable solution is to use centrifugal pumps with mechanical seals and all wetted parts in alloy 20 (N08020).20 Valves Types of valves used in caustic service include globe, gate, ball, plug, diaphragm, or butterfly. Materials used include resistant metals or polymers, often in combination with each other, depending on design and service conditions. Carbon steel, 176 Materials Selector for Hazardous Chemicals nickel or nickel alloys, stainless steel, fluorocarbons, EPDM, and chlorosulfonated polyethers have all been used successfully in different types of valves for caustic. The major concern with valves, aside from mechanical integrity and innate corrosion resistance, is the difference between simple shutoff valves and throttling valves. In a shutoff valve, there are potential problems of crevice corrosion on the seat or closure. In caustic-containing oxidizing species, the oxidant will be consumed within the crevice, setting up a concentration cell effect. When a valve is left partially open, the flow is made more turbulent and erosive and may require more resistant materials. Gaskets, Seals, and O-Rings Gaskets are used to seal the metallic or nonmetallic flange faces of pipes. Most of the gaskets used on caustic soda are based on PTFE. Some of these fluorocarbon plastic gaskets are prevented from flowing by mechanical means; other grades are filled with graphite or other fillers to minimize cold flow. Another gasketing option is an envelope gasket. This consists of a core of an elastomeric core sheathed in a thin sheet of fluoroplastic to resist the caustic soda. PTFE gaskets used with titanium or zirconium should be made from virgin PTFE and not include any recycled product. There have been cases of fluoride corrosion in gasket areas when recycled PTFE gaskets were used. A newer development provides a soft, easily compressible chemically inert 100% PTFE material with a unique combination of chemical resistance and low torque requirements. Most grades of Gylon威 PTFE gaskets are suitable for caustic soda up to 60% at temperatures up to 121⬚C (250⬚F); other grades are appropriate for use in caustic up to 75% at temperatures up to 204⬚C (399⬚F).21 O-ring materials that are compatible with caustic soda include the following:22 • • • • • • Ethylene/tetrafluoroethylene polymer (Viton威TBR-S, Aflas威) Buna-N威 (nitrile) Butyl Ethylene-propylene Perfluoroelastomer (Kalrez威, Chemraz威) Polychloroprene (neoprene) These materials are suitable for static and dynamic seals, within their thermal limits. The following materials have found service as seals, as tapes and gaskets, or as sealing pastes and packing materials in caustic environments: • • • • • • Flexible graphite—concentrated NaOH and for molten NaOH Graphite and carbon Natural rubber Nitrile rubber—to 120⬚C (250⬚F) Polychloroprene—to 105⬚C (220⬚F) Chlorosulfonated PE (Hypalon威) MS-6: Ammonia and Caustic Soda • • • • • 177 Fluorosilicone grease—to 50% NaOH Fluoroplastics Fluoroelastomers (FKM) Butyl rubber cements—limited to 40% NaOH Spiral-wound type 304/PTFE Asbestos gaskets have been commonly used in the past but are now banned or restricted in many locations. Shipping of Caustic Soda Shipping of caustic soda is regulated in most countries. In the United States, the Department of Transportation (DOT) regulates the transport of hazardous materials; these regulations are enforced by different agencies depending on the mode of shipping. Rail shipping is controlled by the Federal Railroad Administration, vessels and water shipping by the U.S. Coast Guard, road shipping by the DOT, and pipeline shipping by the DOT or state regulatory commissions.23 The DOT classification of caustic soda solutions is shown in Table 18.2. The relevant government regulations for both land and sea transport are detailed in Title 49 of Code of Federal Requirements. The DOT documents 111A60W or 111A100W and 407 or 412 define the detail design requirements for the approved shipping containers for rail and highway transport, respectively. Different containers are used to transport caustic soda solutions, depending on the grade of caustic and the temperatures involved. Containers include drums, barrels, tank trucks, cars, and barges. High-quality grades of caustic should not be transported or stored in unprotected steel containers. Drums are usually either steel coated with selected polymeric organic linings or have an inner pack of polyethylene or, for more critical applications, an austenitic stainless steel or solid nickel alloy 200 (N02200). Railroad tank cars and over-the-road tank trucks may be of carbon steel, provided that iron contamination is not a problem. Tank cars are usually coated with ENP or a baked epoxy coating to minimize such contamination. Tank trucks may be of austenitic stainless steel (standard for many commodities) or epoxy coated or rubber lined. 73% NaOH is best handled in alloy 200 (N02200). Table 18.2 DOT Classifications for Caustic Soda Concentration DOT No. Classification Dry, solid Solution 1823 154 1824 154 Non-flammable compressed gas Corrosive material 178 Materials Selector for Hazardous Chemicals For marine transport, barge tanks are either coated with organic coatings or ENP or of type 304 (S30400) or 316 (S31600) construction. The low-temperature curing neoprene latex coatings specified in the past are no longer available. For all types of transport, labeling requirements and handling information are available from suppliers and from regulatory organizations, for example, in the United States, the Interstate Commerce Commission, the Coast Guard, and the International Air Transport Association. References 1. C.P. Dillon, W.I. Pollock, eds. Materials Selector for Hazardous Chemicals: Hydrochloric Acid, Hydrogen Chloride and Chlorine, vol. MS-3 (St. Louis, MO: MTI, 1995), 200 pp. 2. C.M. Schillmoller, “Select the Right Alloys for Caustic Soda Service,” Chemical Engineering Progress May (1996): pp. 48–55. 3. C.M. Schillmoller, “Alloy Selection for Caustic Soda Service,” NiDI technical series no. 10019 (Toronto, ON, Canada: NiDI, March 1988), 9 pp. 4. J.R. Crum, W.G. Lipscomb, “Correlation between Laboratory Tests and Field Experience for Nickel 200 and 26-1 Stainless Steel in Caustic Service,” CORROSION/83, paper no. 23 (Houston, TX: NACE International, 1983), 18 pp. 5. A. Sabata, W.J. Schumacher, “Martensitic and Ferritic Stainless Steels,” in CASTI Handbook of Stainless Steels and Nickel Alloys, ed. S. Lamb (Edmonton, AB, Canada: CASTI Publishing Inc., 2000), p. 149. 6. B. Lundblad, “Akzo Nobel Steps Ahead with New Technology for Caustic Evaporation,” The Alfa Laval International Customer Magazine 9, June (2003): p. 1. 7. F. Smith, Chemetics International (1981), unpublished report. 8. Anon, “Improving Your Product Quality with VenPure Borohydride Products,” Rohm and Haas (2003), http://www.rohmhaas.com/process/process_chem/ app_causticsoda.html. 9. M.P. Sukumaran Nair, “Stress Corrosion Cracking—A Caustic Experience,” Chemical Engineering January (2003): pp. 1–3. 10. Anon, “Sodium Hydroxide Solution and Potassium Hydroxide Solution (Caustic) Storage Equipment and Piping Systems,” pamphlet no. 2, 2nd ed. (Washington, DC: The Chlorine Institute Inc., 2001), 79 pp. 11. API 12F, “Shop Welded Tanks for Storage of Production Liquids” (Washington, DC: API, latest ed.). 12. API 620, “Design and Construction of Large, Welded, Low-Pressure Storage Tanks” (Washington, DC: API, latest ed.). 13. API 650, “Welded Steel Tanks for Oil Storage” (Washington, DC: API, latest ed.). 14. ASME Section V111, “Pressure Vessels” (New York, NY: ASME International, latest ed.). 15. ASME RTP-1, “Reinforced Thermoset Plastic Corrosion Resistant Equipment” (Fiberglass construction) (New York, NY: ASME International, latest ed.). MS-6: Ammonia and Caustic Soda 179 16. J.K. Nelson, “Corrosion by Alkalies and Hypochlorites,” in The Metals Handbook—Corrosion, vol. 13, 9th ed., ed. J.R. Davis (Metals Park, OH: ASM International, 1987), pp. 1174–1180. 17. Anon, “A Guideline for Cleaning & Inspection of Caustic Soda Storage Tanks” (Midland, MI: Dow Chemical Co., 1999), 4 pp. 18. ANSI B31-3, “Chemical Plant and Petroleum Refinery Piping,” ASME Code for Pressure Piping, ASME/ANSI (1990). 19. Anon, “Caustic Soda Storage and Handling” (Midland, MI: Dow Chemical Co., 2003), pp. 19–20. 20. Anon, “General Guidelines for 50% Caustic Soda Storage” (Midland, MI: Dow Chemical Co., 2003), 1 p. 21. Anon, “Engineered Gasketing Products,” DPI-8/01 Rev. 0-5M (Palmyra, NY: Garlock Sealing Technologies, 2001), 56 pp. 22. Anon, “O-Ring Compatibilities,” Engineering Fundamentals, Efunda (2002), http://www.efunda.com/DesignStandards/oring. 23. Anon, “Caustic Soda” (Cleveland, TN: Olin Chlor Alkali Products), 48 pp. Appendix A. Nominal Composition of Alloys Common Name UNS No. Nominal Composition (%) Alloy Steels 1.25 Cr, 0.5 Mo 2.25 Cr, 1 Mo 5 Cr, 0.5 Mo 7 Cr, 0.5 Mo 9 Cr, 1 Mo 3.5 Ni steel 9 Ni steel K11597 K21590 K41545 S50300 S50400 K32025 K81340 1.25 Cr, 0.5 Mo, 0.45 Mn, 0.75 Si, ⬍0.15 C 2.25 Cr, 1 Mo, 0.45 Mn, 0.5 Si, ⬍0.15 C 5 Cr, 0.5 Mo, 0.45 Mn, 0.5 Si, ⬍0.15 C 7 Cr, 0.5 Mo, ⬍1 Mn, ⬍1 Si, ⬍0.15 C 9 Cr, 1 Mo, ⬍1 Mn, ⬍1 Si, ⬍0.15 C 3.5 Ni, 0.9 Mn, 0.28 Si, ⬍0.2 C 9 Ni, ⬍0.9 Mn, 0.23 Si, ⬍0.13 C NiResist威 Alloy Cast Irons Type 1 F41000 Type 2 F41002 Type 3 F41002 Type 5 F41006 Type D2 F43000 Type D5 F43006 15.5 Ni, 2 Cr, 6.5 Cu, 1 Mn, 2 Si, ⬍3 C 20 Ni, 2 Cr, ⬎0.5 Cu, 1 Mn, 2 Si, ⬍3 C 20 Ni, 4.5 Cr, ⬎0.5 Cu, 0.8 Mn, ⬍3 C 35 Ni, ⬍0.1 Cr, ⬍0.5 Cu, 1 Mn, 1.5 Si, ⬍2.4 C 20 Ni, 2.25 Cr, 1 Mn, 2.25 Si, ⬍3 C 35 Ni, ⬍0.1 Cr, ⬍1 Mn, 2 Si, ⬍2.4 C Austenitic Stainless Steels 302 S30200 304 S30400 304L S30403 309 S30900 309S S30908 2RE10 S31002 310 S31000 310L S31050 310S S31008 316 S31600 316L S31603 316Ti S31635 18 Cr, 9 Ni, ⬍0.15 C 18 Cr, 8 Ni, ⬍0.08 C 19 Cr, 10 Ni, ⬍0.03 C 22 Cr, 12 Ni, ⬍0.20 C 22 Cr, 12 Ni, ⬍0.08 C 24.5 Cr, 20.5 Ni, ⬍0.015 C, ⬍0.15 Si 25 Cr, 20 Ni, ⬍0.25 C 25 Cr, 20 Ni, ⬍0.02 C 25 Cr, 20 Ni, ⬍0.08 C 17 Cr, 12 Ni, 2.7 Mo, ⬍0.08 C 17 Cr, 12 Ni, 2.7 Mo, ⬍0.03 C 17 Cr, 12 Ni, 2.7 Mo, ⬍0.08 C, Ti ⬎5 ⳯ C 181 182 Materials Selector for Hazardous Chemicals Common Name UNS No. Nominal Composition (%) 317L 321 347 S31703 S32100 S34700 19 Cr, 13.5 Ni, 3.5 Mo, ⬍0.03 C 18 Cr, 10.5 Ni, ⬍0.08 C, Ti ⬎5 ⳯ C 18 Cr, 11 Ni, ⬍0.08 C, Cb ⬎10 ⳯ C Heat-Resisting Alloys 253 MA S30815 HP N08705 HK-40 J94204 HP-Mod — XTM — DS — 21 Cr, 11 Ni, 1.7 Si, ⬍0.08 C 26 Cr, 36 Ni, ⬍2.5 Si, ⬍2 Mn, ⬍0.5 Mo, 0.55 C 25 Cr, 20 Ni, 1.0 Si, ⬍1.5 Mn, 0.4 C 26 Cr, 35 Ni, 1.5 Si 35 Cr, 48 Ni, 1.5 Si 18 Cr, 11 Ni, 2.2 Si, 0.2 Al, 0.06 C High-Performance Austenitic Alloys 904L N08904 21 Cr, 25.5 Ni, 4.5 Mo, 1.5 Cu, ⬍0.02 C 2RK65 N08904 20 Cr, 25 Ni, 4.5 Mo, 1.5 Cu, ⬍0.025 C 254SMO S31254 20 Cr, 18 Ni, 6.2 Mo, 0.7 Cu, 0.2 N, ⬍0.02 C 25-6MO N08926 20 Cr, 25 Ni, 6.5 Mo, 0.9 Cu, 0.2 N 1925hMo/926 N08926 20 Cr, 25 Ni, 6.2 Mo, 0.8 Cu, 0.2 N, ⬍0.02 C AL-6X N08366 21 Cr, 24.5 Ni, 6.5 Mo, ⬍0.035 C AL-6XN N08367 20.5 Cr, 24 Ni, 6.3 Mo, 0.2 Cu, 0.2 N, ⬍0.02 C 654SMO S32654 24 Cr, 22 Ni, 7.3 Mo, 0.5 Cu, 0.5 N, ⬍0.01 C,3 Mn 20Cb-3 N08020 20 Cr, 35 Ni, 2.5 Mo, 3.5 Cu, 0.07 Cb, ⬍2 Mn, ⬍1 Si, ⬍0.02 C 20 Mod N08320 22 Cr, 26 Ni, 5 Mo, ⬍2.5 Mn, ⬍1 Si, ⬍0.05 C, Ti 800 N08800 20 Cr, 31 Ni, ⬍0.08 C, 0.4 Si, 0.3 Al, 0.4 Ti 825 N08825 21.5 Cr, 42 Ni, 3 Mo, 2.3 Cu, ⬍0.05 C, 0.9 Ti, ⬍0.2 Al Alloy 28 N08028 27 Cr, 32 Ni, 3.5 Mo, 1.0 Cu, ⬍0.03 C Alloy 31 N08031 27 Cr, 31 Ni, 6.5 Mo, 1.2 Cu, 0.2 N, ⬍0.02 C Alloy 33 R20033 33 Cr, Bal Ni, 32 Fe, 1.6 Mo, 0.6 Cu, 0.4 N G N06007 22.5 Cr, Bal Ni, 6.5 Mo, 2 Cu, ⬍0.03 C, 19.5 Fe, 2 Cb, G-3 N06985 22.5 Cr, Bal Ni, 7 Mo, 2 Cu, ⬍0.015 C, 19.5 Fe, ⬍0.5 Cb Ⳮ Ta G-30 N06030 30 Cr, Bal Ni, 5 Mo, 1.7 Cu, ⬍0.03 C, 15 Fe, 2.7 W,⬍5 Co, 0.9 Cb Ⳮ Ta Duplex Stainless Steels 2205 S31803 2304 S32304 2507 S32750 3RE60 S31500 7-Mo — 7-Mo PLUS S32950 — S32906 22 Cr, 5.5 Ni, 3.0 Mo, 0.14 N, ⬍0.03 C 23 Cr, 4 Ni, 0.1 N, ⬍ 0.03 25 Cr, 7 Ni, 4.0 Mo, 0.3 N, ⬍0.03 18.5 Cr, 5 Ni, 2.7 Mo, 0.1 N, ⬍0.03 C 27.5 Cr, 4.5 Ni, 1.5 Mo, ⬍2 Mn, ⬍0.10 C 27.5 Cr, 4.5 Ni, 2 Mo, ⬍2 Mn, ⬍0.03 C 29 Cr, 6 Ni, 2 Mo, 0.4 N, ⬍0.03 C MS-6: Ammonia and Caustic Soda Common Name UNS No. 183 Nominal Composition (%) Ferritic Stainless Steels 409 S40900 430 S43000 434 S43400 444 S44400 446 S44600 E-Brite 26-1威 S44627 XM-27 S44627 Sea-Cure威 S44660 Monit S44635 29-4C S44735 29-4-2 S44800 11 Cr, 0.5 Ni, ⬍0.08 C, Ti 17 Cr, ⬍0.12 C 17 Cr, 1 Mo, ⬍0.12 C 18 Cr, 2 Mo, ⬍0.02 C, Cb/Ti 25 Cr, ⬍0.20 C 26 Cr, 1 Mo, 0.002 C, Cb 26 Cr, 1 Mo, 0.002 C, Cb 27.5 Cr, 1.2 Ni, 3.5 Mo, 0.5 Ti, 0.3 Si 25 Cr, 4.0 Ni, 4.0 Mo, 0.5 Ti, 0.35 Si 29 Cr, 0.3 Ni, 4.0 Mo, 0.5 Ti, 0.35 Si 29 Cr, 2.1 Ni, 4.0 Mo, 0.1 Si Precipitation-Hardening Steels 15-5 PH S15500 17-4 PH S17400 5 Ni, 15 Cr, 3.5 Cu, ⬍1 Mn, ⬍1 Si, ⬍0.07 C 4 Ni, 17 Cr, 4 Cu, ⬍1 Mn, ⬍1 Si, ⬍0.07 C Nickel-Based Alloys Balance is Ni unless Ni content is stated 200 N02200 ⬎99 Ni, ⬍0.25 Cu, ⬍0.4 Fe, ⬍0.35 Mn, ⬍0.02 C 201 N02201 ⬎99 Ni, ⬍0.25 Cu, ⬍0.4 Fe, ⬍0.35 Mn, ⬍0.02 C 230 N02230 ⬎99 Ni, ⬍0.1 Fe, ⬍0.1 Cu, ⬍0.15 Mn, ⬍0.15 C 400 N04400 66.5 Ni, Bal Cu, ⬍0.3 C, ⬍2.5 Fe, ⬍2 Mn,⬍0.5 Si K500 N05500 66.5 Ni, Bal Cu, ⬍2 Fe, 0.27 Al, ⬍1.5 Mn, ⬍0.5 Si, 0.6 Ti, ⬍0.25 C 600 N06600 16 Cr, ⬎72 Ni, ⬍0.5 Cu, ⬍0.15 C, 8 Fe 601 N06601 60 Ni, 22 Cr, ⬍1 Cu, 1.5 Al, ⬍0.1 C 602CA N06025 25 Cr, 9 Fe, 2.2 Al, 0.2 Si, 0.018 C, Ⳮ Y, Zr, Ti 617 N06617 22 Cr, 12.5 Co, 9 Mo, 1.2 Al 625 N06625 22 Cr, 61 Ni, 9 Mo, ⬍0.10 C, ⬍5 Fe, 3.6 Nb 690 N06690 29 Cr, 9 Fe 693 N06693 29 Cr, 4 Fe, 3 Al, 2 Nb, ⬍1 Mn, ⬍ 1 Ti, ⬍0.5 Si, ⬍0.5 Cu, ⬍0.15 C B-2 N10665 ⬍1.0 Cr, 68 Ni, 28 Mo, ⬍0.02 C, ⬍1 Co, 1.8 Fe C-4 N06455 16 Cr, 54 Ni, 15.5 Mo, ⬍0.015 C, ⬍3 Fe, 0.7 Ti, ⬍2 Co C-22 N06022 21 Cr, 13 Mo, 3 W, 4 Fe, 0.2 V, 1.7 Co, 0.003 C C-276 N10276 15.5 Cr, 54 Ni, 16 Mo, ⬍0.02 C, ⬍2.5 Co, 5.5 Fe, 4 W C-2000 N06200 23 Cr, 16 Mo, 1.6 Cu, ⬍0.01 C, ⬍0.08 Si Alloy 59 N06059 23 Cr, 16 Mo, 1 Fe Copper Alloys ETP copper Cartridge brass C11000 C26000 ⬎99.9 Cu 70 Cu, ⬍0.05 Fe, ⬍0.07 Pb, Bal Zn 184 Materials Selector for Hazardous Chemicals Common Name UNS No. Nominal Composition (%) Ounce metal Gunmetal C83600 C90550 85 Cu, ⬍1 Ni, ⬍0.3 Fe, 5 Sn, 5 Zn, 5 Pb, ⬍0.25 Sb 87.5 Cu, ⬍1 Ni, ⬍0.2 Fe, 10 Sn, 2 Zn, ⬍0.3 Pb, ⬍0.2 Sb Titanium Alloys Grade 2 Grade 7 Grade 12 R50400 R52400 R53400 ⬍0.3 Fe, Bal Ti ⬍0.3 Fe, Bal Ti, 0.15 Pd ⬍0.3 Fe, Bal Ti, 0.3 Mo, 0.8 Ni Zirconium Alloys Zirconium 702 Zirconium 704 Zirconium 705 R60702 R60704 R60705 ⬍0.2 Fe Ⳮ Cr, 99.2 Zr Ⳮ Hf, ⬍4.5 Hf 0.3 Fe Ⳮ Cr, 97.5 Zr Ⳮ Hf, ⬍4.5 Hf, 1.5 Sn 0.2 Fe Ⳮ Cr, 95.5 Zr Ⳮ Hf, ⬍4.5 Hf, 2.0–3.0 Nb Cast Alloys CD 4MCu CF 3 CF 3M CF 8 CF 8M CN TM J93370 J93500 J92800 J92600 J92900 N08007 65 69 68 71 68 44 Fe, 25 Fe, 18 Fe, 19 Fe, 19 Fe, 19 Fe, 20 Cr, Cr, Cr, Cr, Cr, Cr, 5 Ni, 2 Mo, 3 Cu, 0.03 C 12 Ni, 1 Si, 0.02 C 10 Ni, 2 Mo, 1 Si, 0.02 C 9 Ni, 1 Si, 0.05 C 10 Ni, 2 Mo, 1 Si, 0.05 C 29 Ni, 2.2 Mo, 1 Si, 3.4 Cu, 0.05 C Appendix B. Approximate Equivalent Grade of Some Cast and Wrought Alloys Structure Austenitic SS Duplex SS Martensitic or ferritic SS Nickel-based alloys Alloy Name Cast (ACI) Cast UNS Wrought 304L 304 316 316L 310 309 Alloy 20 Alloy 2205 CF3, CF3A CF8 CF8M CF3M, CF3MA CK20 CH20 CN7M CD3MN J92500 J92600 J92900 J92800 J94202 J93402 N08007 J92205 — Alloy 410 CD4MCu CA15 J93370 J91150 S30403 S30400 S31600 S31603 S31000 S30900 N08020 S31803 S32205 — S41000 Alloy 420 Alloy 825 CA40 Cu5MCuC J91153 N08826 S42000 N08825 Alloy 600 Alloy 625 Alloy 400 Alloy B-2 CY40 CW6MC M35–2 N7M N12MV CW2M CX2MW CW6M N06040 N26625 N04020 N30007 N30012 N26455 N26022 N30107 N06600 N06625 N04400 N10665 Alloy C-4 Alloy C-22 Alloy C-276 N06455 N06022 N10276 185 Appendix C. Glossary of Corrosion and Materials Terms These corrosion and materials terms have been selected from the “NACE Glossary of Corrosion Related Terms” (Houston, TX: NACE International, 2002, 19 pp.), with permission. active—(1) The negative direction of electrode potential (2) A state of a metal that is corroding without significant influence of reaction product amphoteric—A metal that is susceptible to corrosion in both acid and alkaline environments anion—A negatively charged ion that migrates through the electrolyte anode—The electrode of an electrochemical cell at which oxidation occurs. Electrons flow away from the anode in the external circuit. Corrosion usually occurs, and metal ions enter the solution at the anode anodic protection—Polarization to a more oxidizing potential to achieve a reduced corrosion rate by the promotion of passivity anodizing—Oxide coating formed on a metal surface (generally aluminum) by an electrolytic process austenite—The face-centered cubic structure of iron-based alloys austenitic—A steel in which the predominant structure at room temperature is austenite brittle fracture—Fracture with little or no plastic deformation carbon steel—Alloy of carbon and iron containing up to carbon and up to manganese and residual quantities of other elements, except those intentionally added in specific quantities for deoxidation (usually silicon and/or aluminum) cast iron—Iron-carbon alloy containing approximately 2% to 4% carbon casting (cast component)—Metal that is obtained at or near its finished shape by the solidification of molten metal in a mold cathode—The electrode of an electrochemical cell at which reduction is the principal reaction. Electrons flow toward the cathode in the external circuit 187 188 Materials Selector for Hazardous Chemicals cathodic corrosion—Corrosion resulting from a cathodic condition of a structure, usually caused by the reaction of an amphoteric metal with the alkaline products of electrolysis cathodic protection—A technique to reduce the corrosion of a metal surface by making that surface the cathode of an electrochemical cell cation—A positively charged ion that migrates through the electrolyte toward the cathode under the influence of a potential gradient cavitation—The formation and rapid collapse of cavities or bubbles within a liquid that often results in damage to a material at the solid/liquid interface under conditions of severe turbulent flow corrosion—The deterioration of a material, usually a metal, that results from a reaction with its environment corrosion fatigue—Fatigue-type cracking of metal caused by repeated or fluctuating stresses in a corrosive environment characterized by shorter life than would be encountered as a result of either the repeated or fluctuating stress alone or the corrosive environment alone corrosion inhibitor—A chemical substance or combination of substances that, when present in the environment, prevents or reduces corrosion corrosion potential (Ecorr)—The potential of a corroding surface in an electrolyte relative to a reference electrode under open-circuit conditions (also known as rest potential, open-circuit potential, or freely corroding potential) corrosion rate—The rate at which corrosion proceeds corrosion resistance—Ability of a material, usually a metal, to withstand corrosion in a given system corrosion-resistant alloy (CRA)—Alloy intended to be resistant to general and localized corrosion of oilfield environments that are corrosive to carbon steels corrosiveness—The tendency of an environment to cause corrosion creep—Time-dependent strain occurring under stress crevice corrosion—Localized attack of a metal at or near an area that is shielded from the bulk environment dealloying—The selective corrosion of one or more components of a solid solution alloy (also known as parting or selective dissolution) dezincification—A corrosion phenomenon resulting in the selective removal of zinc from copper-zinc alloys. (This phenomenon is one of the more common forms of dealloying) ductile (nodular) cast iron—Cast iron that has been treated wile molten with an element (usually magnesium or cerium) that spheroidizes the graphite electrochemical cell—A system consisting of an anode and a cathode immersed in an electrolyte so as to create an electrical circuit. The anode and cathode may be different metals or dissimilar areas on the same metal surface electrolyte—A chemical substance containing ions that migrate in an electric field embrittlement—Loss of ductility of a material resulting from a chemical or physical change environment—The surroundings or conditions (physical, chemical, mechanical) in which a material exists MS-6: Ammonia and Caustic Soda 189 environmental cracking—Brittle fracture of a normally ductile material in which the corrosive effect of the environment is a causative factor. Environmental cracking is a general term that includes corrosion fatigue, hydrogen embrittlement, hydrogen-induced cracking (stepwise cracking), hydrogen stress cracking, liquid metal cracking, stress corrosion cracking, and sulfide stress cracking erosion—The progressive loss of material from a solid surface due to mechanical interaction between that surface and a fluid, a multicomponent fluid, or solid particles carried with the fluid erosion-corrosion—A conjoint action involving corrosion and erosion in the presence of a moving corrosive fluid or a material moving through the fluid, leading to accelerated loss of material ferrite—Body-centered cubic crystalline phase of iron-based alloys ferritic steel—Steel whose microstructure at room temperature consists predominantly of ferrite fretting corrosion—Deterioration at the interface of two contacting surfaces under load that is accelerated by their relative motion galvanic corrosion—Accelerated corrosion of a metal because of an electrical contact with a more noble metal or nonmetallic conductor in a corrosive electrolyte graphitic corrosion—Deterioration of gray cast iron in which the metallic constituents are selectively leached or converted to corrosion products, leaving the graphite intact graphitization—The formation of graphite in iron or steel, usually from decomposition of iron carbide at elevated temperatures. (Should not be used as a term to describe graphitic corrosion) heat-affected zone (HAZ)—That portion of the base metal that is not melted during brazing, cutting, or welding but whose microstructure and properties are altered by the heat of these processes heat treatment—Heating and cooling a solid metal or alloy in such a way as to obtain desired properties. (Heating for the sole purpose of hot working is not considered heat treatment) hydrogen blistering—The formation of subsurface planar cavities, called hydrogen blisters, in a metal resulting from excessive internal hydrogen pressure. Growth of near-surface blisters in low-strength metals usually results in surface bulges hydrogen embrittlement—A loss of ductility of a metal resulting from absorption of hydrogen hydrogen-induced cracking—Stepwise internal cracks that connect adjacent hydrogen blisters on different planes in the metal or to the metal surface (also known as stepwise cracking) inhibition—To inhibit means to retard or slow the rate of corrosion, usually by the addition of other chemicals to the system intergranular corrosion (IGC)—Preferential corrosion at or near the grain boundaries of a metal iron rot—Deterioration of wood in contact with iron-based alloys knife-line attack (KLA)—Local corrosion along a line adjacent to a weld after heating into the sensitization temperature range 190 Materials Selector for Hazardous Chemicals liquid metal cracking (LMC)—Cracking of a metal caused by contact with a liquid metal low-alloy steel—Steel with a total alloying element content of less than about 10% but more than specified for carbon steel metallizing—The coating of a surface with a thin metal layer by spraying, hot dipping, or vacuum deposition oxidation—(1) Loss of electrons by a constituent of a chemical reaction. (2) Corrosion of a metal that is exposed to an oxidizing gas at elevated temperatures passivation—a reduction in the anodic reaction rate of an electrode involved in a corrosion process passive—(1) The positive direction of electrode potential. (2) A state of a metal in which a surface reaction product causes a marked decrease in the corrosion rate relative to that in the absence of the product pH—The negative logarithm of the hydrogen ion activity written as: pH ⳱ ⳮlog10(aHⳭ), where aHⳭ ⳱ hydrogen ion activity ⳱ the molar concentration of hydrogen ions multiplied by the mean ion-activity coefficient pitting—Localized corrosion of a metal surface that is confined to a small area and takes the form of cavities called pits pitting factor—The ratio of the depth of the deepest pit resulting from corrosion divided by the average penetration as calculated from mass loss rust—Corrosion product consisting of various iron oxides and hydrated iron oxides. (This term properly applies only to iron and ferrous alloys) sensitization—Precipitation of constituents (usually carbides) in a structure as a result of heating and cooling through a certain temperature range. Can lead to intergranular corrosion stress corrosion cracking (SCC)—Cracking of metal involving anodic processes of localized corrosion and tensile stress (residual and/or applied) sulfidation—The reaction of a metal or alloy with a sulfur-containing species to produce a sulfur compound that forms on or beneath the surface of the metal or alloy transpassive—The noble region of potential where an electrode exhibits a higherthan-passive current density weld (verb)—To join two or more pieces of metal by applying heat and/or pressure with or without filler metal; to produce a union through localized fusion of the substrates and solidification across the interfaces weld decay—Intergranular corrosion associated with sensitization due to welding weldment—That portion of a component on which welding has been performed, including the weld metal, the heat-affected zone (HAZ), and the base metal weld metal—That portion of a weldment that has been molten during welding wrought metal—Metal in the solid condition that is formed to a desired shape by working (rolling, extruding, forging, and so on), usually at an elevated temperature yield strength—Stress at which a material exhibits a specified deviation from the proportionality of stress to strain Appendix D. Glossary of Acronyms and Abbreviations AAR—Association of American Railroads ACGIH—American Conference of Governmental Industrial Hygienists ANSI—American National Standards Institute AOD—Argon Oxygen Decarburization AP—Anodic Protection API—American Petroleum Institute ASME—American Society of Mechanical Engineers ASTM—American Society for Testing and Materials BAT—Best Available Techniques BP—Boiling Point BSI—British Standards Institution CAF—Compressed Asbestos Fiber CAS—Chemical Abstracting Service CFR—Code of Federal Regulations CPVC—Chlorinated Polyvinyl Chloride CR—Corrosion Rate ⬚Bé—Degree Baumé DBT—Ductile-Brittle Transition DO—Dissolved Oxygen DOT—Department of Transportation (US) ECTFE—Ethylene Chlorotrifluoroethylene EEC—European Economic Community EFMA—European Fertilizer Manufacturers Association ENP—Electroless Nickel Plating ESC—Environmental Stress Cracking (plastics) ESR—Electroslag Remelting EU—European Union (formerly, European Community, EC) FEP—Fluorinated Ethylene Propylene FKM—Fluorohydrocarbon Elastomers 191 192 Materials Selector for Hazardous Chemicals FP—Freezing Point FRP—Fiber-Reinforced Plastic GRP—Glass Fiber–Reinforced Plastics HAC—Hydrogen-Assisted Cracking HAZ—Heat-Affected Zone HBN—Hardness Brinell Number HDPE—High-Density Polyethylene HRC—Hardness Rockwell C IDLH —Immediately Dangerous to Life or Health IFA—International Fertilizer Industry Association IGA—Intergranular Attack IGC—Intergranular Corrosion KLA—Knifeline Attack LMC—Liquid Metal Cracking LME—Liquid Metal Embrittlement MSDS—Material Safety Data Sheet NDE—Nondestructive Examination NDTT—Nil Ductility Transition Temperature NIOSH—National Institute for Occupational Safety and Health OEL—Occupational Exposure Limit OSHA—Occupational Safety and Health Administration PE—Polyethylene PEL—Permissible Exposure Limit PFA—Perfluoroalkoxy PP—Polypropylene PTFE—Polytetrafluorethylene PVC—Polyvinylchloride PVDC—Polyvinylidene Chloride PVDF—Polyvinylidene Fluoride PVF—Polyvinyl Fluoride PWHT—Postweld Heat Treatment REL—Recommended Exposure Limit SBR—Styrene Butyl Rubber SCC—Stress Corrosion Cracking Sp.Gr.—Specific Gravity SS—Stainless Steel STEL—Short-Term Exposure Limit. TLV—Threshold Limit Value TWA—Time-Weighted Average. Index Locators in italics refer to tables or figures. ABS (acrylonitrile-butadiene-styrene), 48, 139 Acrylic, 139 Acrylonitrile-butadiene-styrene (ABS), 48, 139 Acrylonitrile rubber (Buna-N威), 47, 48, 69, 137, 138, 176 Advanced membrane gap cells (MGPs), 88 Aflas威, 176 AL 29-4-2威 (S44800), 31, 107, 108 AL 29-4C威 (S44735), 31, 107, 108 Alloy 3RE60 (S31500), 32, 56, 65–66 Alloy 6XN (N08367), 117 Alloy 7-Mo威, 110 Alloy 17-4PH, 109 Alloy 20 (N08020), 107, 108, 165, 175 Alloy 20Cb3威 (N08020), 115, 152 Alloy 21/4% Cr, 1%Mo (K21950), 108 Alloy 25-25-2, 122 Alloy 26-1, 146, 147, 148, 165 Alloy 26-1S (S44626), 108 Alloy 28 (N08028), 118, 120, 120, 149, 152 Alloy 29-4 (S44700), 169 Alloy 33威 (R20033), 120, 122, 123, 149, 150 Alloy 200 (N02200): in ammonia service, 39, 39; in caustic soda service, 110, 122, 123, 123–24, 125, 126, 146, 148, 148, 149, 150, 152, 164, 165, 168, 169, 170, 171, 177; in KOH service, 160 Alloy 201 (N02201), 122, 123, 123, 124, 127, 153, 163, 164, 169, 170 Alloy 214, 36, 38 Alloy 230 (N02230), 38 Alloy 254 SMO (S31254), 115, 117 Alloy 400 (N04400): in ammonia service, 40; in caustic soda service, 96, 121, 124, 125, 125, 128, 150, 151, 152, 165, 168, 168, 170, 171, 174; in KOH service, 160 Alloy 446 stainless steel, 38 Alloy 556, 38 Alloy 600 (N06600): in ammonia service, 38, 65; in caustic soda service, 121, 124, 125, 125, 126, 127, 128, 152, 153, 155, 165, 168, 169; chromium equivalents, 36; isocorrosion curves, 127 Alloy 601 (N06601), 35, 36, 36, 38, 128 Alloy 602CA (N06025), 36 Alloy 617 (N06617), 36, 38 Alloy 620, 36 Alloy 625 (N06625): in ammonia service, 35, 39; in caustic soda service, 128, 152, 155, 165, 169, 171 Alloy 654 SMO (S32654), 117, 150 Alloy 690 (N06690), 36, 121, 126, 127, 165 Alloy 693 (N06693), 36 Alloy 800 (N08800): in ammonia service, 36, 36, 63; in caustic soda service, 115, 116, 118, 120, 126, 149, 154, 165 Alloy 800/800HT, 37, 38 Alloy 800H (N08800), 36, 36, 38, 62 Alloy 825 (N08825), 115, 154 Alloy 904 (N80904), 114 Alloy 904L (N08904), 117, 118, 120 Alloy 926 (N06696), 150 Alloy 2205 (S31803), 32, 110 Alloy 2205 Code Plus 2, 117 Alloy 6030 (N06690), 128 Alloy 6375, 48, 138 Alloy APM, 36 Alloy B-2 (N106650), 126, 165 Alloy C-4 (N06455), 128, 150 Alloy C-276 (N10276), 39, 128, 165 Alloy CF3 (J92700) cast alloy, 33–34, 114 Alloy CF3M (J92800), 34, 114 193 194 Alloy CF8 (J92600) cast alloy, 33–34, 114, 115 Alloy CF8M (J92900), 34, 68, 69, 114, 175 Alloy CN7M (J92700), 119, 175 Alloy CN7M (N08007), 117 Alloy CY40 (equivalent N06600), 175 Alloy CZ100 (equivalent N02200), 175 Alloy DS, 36 Alloy ELI 21-1 (S44626), 169 Alloy HK 40 (J94204), 33, 34, 36, 60, 62 Alloy HK 45Nb, 62 Alloy HP (N08705), 34, 60, 62 Alloy HP 45Nb, 34 Alloy HP-Mod, 36, 62, 63 Alloy HT, 62 Alloy HU, 62 Alloy K-500 (N05500), 125 Alloy M35 (equivalent N04400), 175 Alloy S, 38 Alloys, chromium equivalents, 36 Alloy S32906, 148, 149 Alloy SAF 2304 (S32304), 117 Alloy SAF 2507 (S32507), 117 Alloy X, 38 Alloy X1CrNiMoN, 122 Alumina (Al2O3), 151 Aluminum (Al): bauxite refining and, 151–52; caustic soda service, 99; corrosion, 20, 23–24; hydrogen ion liberation, 19; LME risk, 96, 150 Aluminum alloys, 23–24, 96, 99, 150 Aluminum oxide, 144 Aminodiisopropanol, 151 Ammonia, 30%, 9 Ammonia (CAS 7664-41-7): aluminum and, 23–24; carbon steels and, 25–30; cast iron and, 24–25; chemical properties, 9; commercial grades, 7; corrosion by, 19–22; dissociation, 27; health and safety, 9–11; ignition, 11; industrial uses, 4; nitrogen fixation and, 3; physical properties, 8; physiological responses to, 10; production, 13–17, 14, 59–73, 61; properties, 7–12; SCC mitigation, 28; synthesis, 64–65 Ammonia converters, 38 Ammonia plant, 60 Ammonia solutions, 8 Ammonium bicarbonate, 55 Ammonium carbonates, 55 Ammonium chloride, 55 Ammonium hydroxide (CAS 1336-21-6), 3, 7–8, 24, 39, 40, 56–57 Aqua ammonia, 9 Index Asbestos, compressed fibers, 69 Asbestos gaskets, 177 ASTM A213, 64, 65 ASTM A312, 64 ASTM A331, 64 ASTM A335, 64 ASTM materials, 64 Austenitic alloys, high-performance, 115–21 Austenitic nickel cast irons, 101 Austenitic stainless steels, 21, 32–33, 106, 111–13, 153. See also specific alloys; specific types Baffles, 72 Basch, Karl, 13 Bauxite refining, 151–52 Bayer process, 152 Bisphenol-A fumarate, 50, 141, 141 Boilers, 168 Bolting, 70 Borosilicate glass, 143, 143, 144 Brine circulation piping, 169 Brine pumps, 168 Budatiene-styrene rubber (Buna-S威), 47 Buna-N威 (acrylonitrile rubber), 47, 48, 69, 137, 138, 176 Buna-S威 (budatiene-styrene rubber), 47 Butadiene, 138 Butadiene acrylonitrile (Buna-N威), 47, 48, 69, 137, 138, 176 Butyl, 176 Butyl, grade 1, 138 Butyl rubber, 69 Carbon, 52, 141–42, 176 Carbon dioxide, 23 Carbon dioxide, carbamates, 55–57 Carbon dioxide removal system, 64 Carbon steels: in ammonia service, 25–30, 40, 167; in caustic soda service, 101–6, 164 Carburization, 35, 37, 62 Cast alloys, 36. See also specific alloys Cast duplex steels, 117 Cast irons: in ammonia service, 24–25, 25; in caustic soda service, 100–101, 102, 103, 125; gray, 95, 100, 102, 103; gray corrosion, 103; high-silicon, 101; SG (spheroidal graphite), 152; white, 24 Cast stainless steels, 33–34, 113–14 Cathodic protection, 26 Caustic cracking, 113 Caustic dilution, 85–86 Index Caustic fusion reactions, 152–53 Caustic potash. See Potassium hydroxide (KOH) Caustic soda (CAS 1310-73-2, NaOH): boiling points, 82; chemical properties, 82–83; contaminants, 145–51; contamination by, 154; corrosion, 93–97; DOT classifications, 177; handling, 170–77; health and safety considerations, 83–84; impurities, 90–91; physical properties, 81–82, 82, 83; production, 87–92, 88–90, 91, 167–79; protective equipment, 84; SCC and, 21; shipping, 177–78; solutions, 84; treatments, 151–54; uses, 78 CD-4MCu (J93370), 117, 119, 175 Centrifugal pumps, 95 Centrifuges, 168 Ceramic materials, 52, 142–43 CGA pamphlet G-2.1, 71 Chemraz威, 176 Chlor-Alkali plant, 77 Chlorates, 146, 146–48 Chlorides, 20, 21, 33, 57, 148–49 Chlorinated polyester, 141 Chlorinated polyether, 139 Chlorinated polyvinyl chloride (CPVC), 48, 50, 139, 139, 140 Chlorinated rubber, 137 Chlorine/hypochlorite, 149 Chlorine production, 87, 167 Chloroprene (CR, neoprene), 48 Chlorosulfonated polyethylenes (CSPE), 48, 137, 138, 176 Chromium, 113, 118 Chromium-bearing alloys, 126–28 Chromium carbide precipitation, 21 Chromium-molybdenum steels, 28–29, 30 Coal feedstock plant, 60 Code of Federal Regulations (CFR), 71 Cold work, 104 Compressed asbestos fiber (CAF), 69 Compressors, 68–69 Condensers, 66 Contamination: caustic, 154; caustic soda service, 145–57; chlorides, 57; sodium hydroxide, 90–91; of steam, 154–55 Coolers, 66, 171 Copper, 129 Copper alloys, 21, 40, 41, 66, 96, 150 Copper (Cu), 96, 125, 128–29, 150 Corrosion: aluminum, 23–24; by ammonia, 19–22; cast iron, 102; by caustic soda, 93–97; forms of, 20–22, 94–96; of metals and alloys, 99–136 195 Corrosion-resistant alloys (CRAs), 115, 121 CPR, 69 CPVC (chlorinated polyvinyl chloride), 50, 139, 139 Creep resistance, 35 Crevice corrosion, 21 Crystallizers, 168, 169–70 CSM, 138 CSPE (chlorosulfonated polyethylenes), 137 Cupronickel 70-30 (C71500), 129 Cupronickel 90-10 (C70600), 129 Cuprous oxide, 41 Dealloying, 22, 95 Decarburization, 27, 29 Department of Transportation, 70–71, 71, 177–78 Desulfurization section, 59 Diabon F100威, 52 Diaphragm cell process, 88–89 Disposal: ammonia, 11; caustic soda, 85 Distillation columns, 65 Drums, 177 Ductile cast iron (DI), 101 Duplex alloys, 106, 109–11, 111, 148, 152. See also specific alloys Duplex stainless steels, 31–32 Ebonite (hard rubber), 137 E-Brite威 (S44627), 107, 108, 108, 110, 147, 148, 149, 165, 169 ECTFE (Halar威, ethylene chlorofluoroethylene), 48, 50, 140 EFTE (ethylene trifluoroethylene), 50 Elastomers, 47, 48, 137–38, 138. See also specific elastomers Electroless nickel plating (ENP), 88, 126, 175, 178 Electrolytic cells, 91 ELI 21-1 (S44626), 169 Environmental stress cracking (ESC), 139 EPDM (ethylene propylene diene monomer rubber), 47, 48, 69, 137 Epoxy, 50, 51, 88, 141 Erosion-corrosion, 21, 95 ETFE (Tefzel威, ethylene trifluoroethylene), 48, 50, 140, 176 Ethylene chlorofluoroethylene (Halar威, ECTFE), 48, 50, 140 Ethylene propylene, 48, 176 Ethylene propylene diene monomer rubber (EPDM), 47, 48, 69, 137 196 Ethylene trifluoroethylene (ETFE, Tefzel威), 48, 50, 140, 176 ETP-S, 48, 69, 138 Evaporators, 92, 168, 169–70 Explosions, 84 Eye protection, 10–11 F138, 112 Federal Railroad Administration, 177 FEP (fluorinated ethylene propylene), 48, 50, 139, 140 Ferritic stainless steels, 31, 106–9 Ferrous alloys, 100. See also specific alloys Fertilizers, ammonia in, 3 FFKM, 48, 138 Fiber-reinforced plastic (FRP), 49, 50, 50, 51, 140–41, 169 Filters, 168 Fire, 84 First aid, 85 Fittings, 168 FKM, 47, 138 Flexible graphite, 69, 176 Fluorinated ethylene-propylene (FEP), 50, 139, 140 Fluorocarbon elastomers, 137 Fluoroelastomers, 48, 138 Fluorohydrocarbon elastomers, 47 Fluorosilicone, 137 49 CFR 173.314, 71 FPA (perfluoro alkoxy), 50 FRP (fiber-reinforced plastic), 49, 50, 50, 51, 69, 140–41 Furane (furfural-furfuryl alcohol), 50, 141, 141 Galvanic corrosion, 95 Gaskets, 69, 176–77 General corrosion, 94–95 Glass, 52, 52, 69, 142, 142–43 Glycolate, 155 Grain boundary embrittlement, 152 Grain size, corrosion and, 42 Graphite, 52, 69, 141–42, 176 Graphitization, 122 Gray cast iron, 95, 100, 102 Gunmetal (C90550), 129 Gylon威 PTFE gaskets, 69, 176 Haber, Fritz, 13 Haber-Bosch process, 13 Halar威 (ECTFE, ethylene chlorofluoroethylene), 48, 50, 140 Index Hard rubber (Ebonite), 69, 137 Heaters, 65–66, 168, 171 Heat exchangers, 65–66, 168, 171 Heat recovery steam generators (HRSGs), 155 Heat transfer, 96 Hexoloy威 (silicon carbide), 143, 144 High-density polyethylene (HDPE), 138, 139 High-phosphorus EN (HPEN), 126 High-silicon cast irons, 101 High-temperature converter, 64 High-temperature corrosion, 22, 96 Hoses, 69 Hydrogenated bis-A-polyester, 141 Hydrogen attack, 27–29 Hydrogen chloride (HCl), 55 Hydrogen embrittlement, 64 Hydrogen service, 29 Hydrogen sulfide, 23, 29–30, 35, 152 Hypalon威, 48, 138, 176 Hypochlorites, 146, 149 Iconel, 161 Ingestion, ammonia, 10 Intergranular attack (IGA), 21, 95, 108, 123, 127, 169 Iron, 24, 100, 150–51 Isobutylene isoprene, 137 Isocorrosion curves: 904L, 114; alloy 28, 120; alloy 201, 127; alloy 600, 127; CF8, 115; CN 7M, 119; type 304, 113; type 316, 113 Isophthalates, 141 Isoprene, 47, 69 Kalrez威, 48, 138, 176 KFM, 48 KHR 35 CT, 63 Kynar威 (polyvinylidene fluoride, PVDF), 48, 139 Lead, 43 Leaks, 11, 85 L grade stainless steels, 112 Linings, 172 Liquid metal embrittlement (LME), 96, 125, 127, 129, 130, 150 Localized corrosion, 95 Low-alloy steels, 101–6 Low-C steel, 161 Low-phosphorus EN (LPEN), 126 Magnesium, 43 Magnetite, 100 Index Martensitic stainless steels, 106 Material Safety Data Sheets (MSDA), 71 Medium-phosphorus EN (MPEN), 126 Membrane cell process, 89–90, 90 Mercury, 149–50 Mercury cell process, 89 Metal dusting, 35–37, 63–64 Metal finishing, 153 Metal wastage rates, 37 Methyl diethanolamine (MDEA), 64 Mid American Pipeline System (MAPCO), 72 Mild steel, 125 Molybdenum, 116, 118 Monel, 161 Monit威 (S44635), 31, 107 N08904, 112 Natural rubber (NR), 47, 48, 69, 137, 138 NBR, 48, 138 Nelson curves, 27 Neoprene (chloroprene CR), 47, 48, 137, 176 Neoprene latex, 137, 178 NHT (naphtha hydrotreater), 55 Nickel: caustic soda service, 121–28; corrosion and, 102, 116, 161; effects of, 116, 117, 118, 121 Nickel alloys: in ammonia service, 30, 37–39; in caustic soda service, 121–28, 165, 168; effects of, 164; metal wastage rates, 37; nitriding depth and, 38; nitrogen absorption, 38. See also specific alloys Nil Ductility Transition Temperature (NDTT), 33 Niobium, 43 NIOSH/MSHA acid-gas respirators, 10–11 NiResist威, 25, 102, 103, 151, 168, 169; type 1 (F41000), 101, 125; type 2 (F41002), 101, 125; type D2 (F3000), 101 Nitriding, steel, 27 Nitriding depth, 38 Nitriding tests, 38 Nitrile rubbers, 48, 137, 138 Nitrogen absorption, 38 Nitrogen fixation, 3 Nonmetallic materials: in ammonia service, 47–53; in caustic soda service, 137–44 Nordel威, 48 1050LF, 48, 138 O-rings, 69, 70, 176–77 Oxide layers, 19–20, 94 Paper operations, 153–54 197 Partial oxidation process, 15 Passivity, 19–20, 93–94 Perfluoro alkoxy (PFA), 50, 140 Perfluoroelastomers, 47, 48, 137, 138, 176 Petroleum refining, 151 PE/vinyl acetate (PVA), 139 PFA (perfluoro alkoxy), 48, 140 PH, 19, 24, 82 Phenolic resins, 141 Phosphorus, 126 Pipelines, 72, 168 Piping, 68, 129 Pitting corrosion, 20 Plastic-lined pipe, 49 Plastics, 47–52, 138–41 Polybutylene (PB), 48 Polycarbonate, 139 Polychloroprene (neoprene), 137, 176 Polychloroproprene rubber, 47 Polyesters, 48 Polyethylene (PE), 48, 50, 139, 140 Polyisobutylene, 48 Polypropylene (PP), 48, 50, 138, 139, 140 Polystyrene, 48, 139 Polysulfide, 138 Polytetrafluoroethylene (PTFE, Teflon威), 48, 138, 139, 140, 176 Polyurethane, 141, 141 Polyvinyl chloride (PVC), 48, 49, 50, 139, 139, 140 Polyvinylidene chloride (PVDC), 139 Polyvinylidene fluoride (PVDF), 48, 50, 139, 139, 140 Postweld heat treatment (PWHT), 29, 67, 154, 172 Potassium chlorate (KClO3), 160, 161 Potassium chloride (KCl), 160 Potassium hydroxide (KOH, CAS 1310-58-3), 78, 113, 128, 160–61 Precipitation-hardening (PH), 30, 31, 106, 109 Pressure vessels, 167–69 Pressurized pressure vessels, 67–68 Primary reformer, 60–63 Production equipment: ammonia, 59–73; caustic soda, 167–79 Protective equipment: ammonia, 10–11; caustic soda, 84 PTFE (Teflon威, polytetrafluoroethylene), 48, 48, 69, 139, 176 Pulp operations, 153–54 Pumps, 68, 168, 175 PVC (polyvinyl chloride), 48, 49, 50 198 PVDF (Kynar威, polyvinylidene fluoride), 48, 48, 139 PWHT (postweld heat treatment), 29, 67, 154, 172 Railcar transport (tank cars), 71–72 Refrigerants, 3 Refrigerated storage tanks, 66–67 Rubber, 47, 69, 137, 138 Ruwais Fertilizer Industries (FERTIL), 63 Salt separators, 170 SCM, 48 Sea-Cure威 (S44660), 31, 107, 147, 148, 149 Seals, 69, 176–77 Secondary reformers, 63–64 Settling tanks, 168 SG (spheroidal graphite) cast iron, 152 Shomac威 30-2, 107 Showa Denko, 16–17 S-phase precipitation, 31 Silica, 69 Silicon carbide (Hexoloy威), 143 Silicon cast irons (F47003), 101 Silicone, 138 Silicone rubber, 137 Silver, 43, 131 Skin, 10–11 Soap manufacture, 152 Soda ash, 153, 159–60 Sodium, molten, 155 Sodium bicarbonate (NaHCO3), 155, 156 Sodium carbonate (Na2CO3, CAS 497-19-8). See Soda ash Sodium chlorate, 147 Sodium chloride, 147 Sodium hydrosulfide (NaSH), 152 Sodium hydroxide (NaOH). See Caustic soda Sodium hypochlorite, 169 Sol-gel procedures, 34–35 Solvay (ammonia soda), 155 Spills: ammonia, 11; caustic soda, 85 Stainless steels: in ammonia service, 30–34; cast, 113–14; in caustic soda service, 106, 163, 164; corrosion, 20, 107, 108, 122; IGA in, 21; isocorrosion curves, 113, 114, 120; L grade, 112. See also specific types Static corrosion rates, 124 Steam, contamination of, 154–55 Steam/air reforming process, 13, 14 Index Storage tanks, 66–68, 171–73 Stress corrosion cracking (SCC), 96, 127; austenitic stainless steels, 153–54; in caustic soda service, 100, 104, 105, 122; chloride, 31, 33, 172; chromium-bearing alloys, 126–28; description, 21; isocorrosion curves, 113, 114; mitigation measures, 28; nickel alloys and, 121; soda ash and, 155; soil subsidence and, 173; steam contamination and, 154; steel, 26–27 Sulfides, 153 Sulfur, 150 Surface passivity, 93–94 T22 material, 65 Tank cars, 71–72, 168, 177 Tanks, 28 Tank trucks, 72, 177 Tantalum, 43, 131–32 Tantalum-tungsten alloy, 132 TBR-S, 48, 69, 138, 176 Teflon威 (polytetrafluoroethylene, PTFE), 48, 48, 69, 138, 139, 140 Tefzel威 (ETFE, ethylene trifluoroethylene), 48, 50, 140 Temperature: alloy use and, 34–37; caustic soda service and, 100–101, 105; corrosion and, 107, 108, 117; limits, 48, 49; nitriding depth and, 38; SCC and, 105 Temper embrittlement, 29 Thermoplastics, 48–49, 138–40, 139 Thermoset resins, 49–51, 140–41 Tin, 43 T-I steel (A-517), 72 Titanium alloys, 42, 129–30 Titanium (R50400): in ammonia service, 42, 69; in caustic soda service, 96, 129–30, 130, 150, 176; in KOH, 161 Title 29 (Labor), 71 Title 33 (Navigation and Navigable Waters), 71 Title 40 (Protection of the Environment), 71 Title 46 (Shipping), 71 Title 49 (Transportation), 71 Transfer piping, 168 Transportation equipment, 70–72, 177–78 Triple-Effect Caustic Soda Evaporator, 92 Trona, 155 Tungsten carbide, 144 Type 302 (S30200), 32, 111 Type 304 (S30400), 107, 107; in ammonia ser- Index vice, 32, 33, 34, 38, 56, 60, 63, 64, 65, 67, 68, 69, 71; in caustic soda service, 108, 110, 111, 112, 114, 117, 155, 156, 164, 165, 178; chromium equivalents, 36; desulfurization, 59; isocorrosion curves, 113. See also Alloy CF8 (J92600) cast alloy Type 304L (S30403), 32, 68, 111, 112, 113, 114, 118, 153, 154, 171, 174. See also Alloy CF3 (J92700) cast alloy; Isocorrosion curves Type 309 (S30900), 38 Type 310 (S31000), 34, 36, 37, 38, 60 Type 310S (S31008), 33 Type 316 (S31600): in ammonia service, 33, 56, 64; in caustic soda service, 108, 122, 148, 148, 164, 165, 178; desulfurization, 59; isocorrosion curves, 113 Type 316L (S31603), 32, 108, 111, 112, 117, 118, 118, 120, 151, 152, 154, 174 Type 316Ti (S31635), 32, 112, 120–21, 150 Type 317 (S31700), 33, 112 Type 317L (S31703), 33, 108, 112, 118 Type 321 (S32100), 32, 33, 59, 65, 112, 154 Type 347 (S34700), 32, 34, 60, 112, 155, 160 Type 410 (S41000), 56, 68, 69, 109 Type 439 (S43035), 108 Type 444 (S44400), 108 199 Type 446 (S44600), 108 Type XM-27 (S44627), 31, 107, 108 Valves, 69, 168, 175–76 Vapor-phase attack, 21–22 Vinyl ester, 50, 51, 141 Viton威, 47, 48, 138, 176 Viton威 A, 69 Viton威 Extreme, 69 Waste heat recovery system, 64 Welding, 32, 104, 172 Weld seams, 27 XTM, 36 Yellow brass (C26800), 41, 42 Zinc, 43, 95, 125 Zinc spraying, 26 Zirconia, 130 Zirconium alloys, 43 Zirconium (R60702): in ammonia service, 43, 69; in caustic soda service, 96, 130–31, 131, 149, 150, 176; in KOH, 161, 161 Zirconium Zr702 (R60702), 43, 130