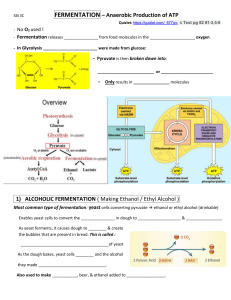
FERMENTATION INDUSTRY OF ALCOHOL LECTURE-6 COURSE CODE: CHM-3112/512 COURSE TITLE: APPLIED CHEMISTRY-II COURSE INCHARGE: DR. FARZANA NAZ INTRODUCTION TO THE TOPIC Fermentation naturally occurs in yeast and some bacteria. Alcohol is commercially produced by using yeast. During fermentation, sugar is anaerobically converted into ethanol, water, and carbon dioxide. Louis Pasteur first worked on the living identity of yeast and its capability to convert fruit sugar into alcohol (alcoholic fermentation). He also worked lactic acid production by bacteria (lactic acid fermentation). In today’s time the science that deals with fermentation is called zymology. CONTENTS OF THE LECTURE Introduction to Fermentation Industry Uses Raw Material Manufacturing Process Batch and Continuous Process OBJECTIVES AND LEARNING GOALS By the end of this lecture you should be familiar with: Fermentation Industry Raw materials and utilizing of the byproducts Manufacturing process of Alcohols by using Batch and Continuous Process BUILDING ON (Prerequisites) Introduction of Sugar Industry Raw Material Byproducts Manufacturing Process Raw & Refined Sugar Factors Affecting Sugar Storage INTRODUCTION Fermentation naturally occurs in yeast and some bacteria. Alcohol is commercially produced by using yeast. During fermentation, sugar is anaerobically converted into ethanol, water, and carbon dioxide. Two types of fermentations are known in first sugars from fruit get fermented which is known as alcoholic fermentation. Whereas, production of lactic acid by bacteria is called lactic acid fermentation. In today’s time the science that deals with fermentation is called zymology. Chemically alcoholic fermentation is divided into two phases: In the first phase the glucose (sugar) is converted into pyruvate by glycolysis. The second phase involves the conversion of pyruvate in alcohol. So basically, the second phase is the fermenting step of the reaction. ALCOHOLIC FERMENTATION Ethanol is obtained by the fermentation process where the sugar content in fruit juices and honey of crops are transformed into alcohol using yeast. Fermentation is mostly feasible for countries where sugar cane is produced at a large extent. Ethanol production can largely be increased if fermentation process is updated. For making beers and lagers, barley is frequently used and for making wines the sugar source is crushed grapes. To increase the alcoholic contents of the beverage, distillation is done because yeast cannot tolerate high levels of alcohol and die. In the process of distillation, fermented solution is heated to vaporize ethanol at the temperature around 78.5 ˚C and after that ethanol vapors are collected and condensed in another flask to get concentrated ethanol. Uses Alcoholic fermentation produces all different types of alcoholic beverages, where the type of beverage depends on the source of sugar and final alcohol content. The alcoholic beverages that are produced by distillation are vodka, rum, and other spirits. Alcoholic fermentation has another application in the bread making industry, where yeast is mixed with dough for the fermentation process. The only difference here is unlike beverages, the carbon dioxide produced during reaction makes the dough rise and ethanol evaporates from it. Ethanol is mostly used as fuel and has become an alternative of renewable energy source now-a-days. Ethanol is an eco-friendly alternative to petroleum-based fuel as it has fewer greenhouse gas emissions. The production of ethanol is growing day by day at a great extent for its versatile application and demand. As the supply and price of oil and gas worldwide has become a major problem, ethanol is taking place as an alternative. Worldwide ethanol production as fuel reached 32.35 billion gallons in 2012. Raw Material SUGAR BEET Mostly preferred 15% sugar, 82% water, small amount of starch Juice is extracted by crushing A ton yield 20 – 25 gallons of alcohol Enzymes improve the alcohol yield SUGAR CORN WASTE 7 – 15% sugar Sugar is extracted as same as sorghum 8 – 18% gallons of alcohol per ton Back slopping in the range of 20-25% Acidification is necessary Sugar beet sugar corn PRODUCTION PROCESS OF ALCOHOL Production process of alcohol is carried out in 3 stages 1. Inoculum 2. Proper fermentation 3. Recovery Preparation of Inoculum A small amount of material containing bacteria, viruses, or other microorganisms that is used to start a culture is called inoculum. After selection of desired organism in its pure form the inoculum is prepared under aseptic condition. For this condition organism is first cultured in flask to increase the size of the inoculum when increases the size of the inoculum which can then be used for inoculation. Manufacturing Process Depending on the extraction process of molasses from different sources, compositions can be varied. Reaction involved Two reactions involve with this process, one is for fermentation and another is for conversion of fructose to ethanol. Catalyst of fermentation reaction is invertase enzyme from yeast and the Catalyst of ethanol conversion reaction is zymase. Previously Batch fermentation was used but now Continuous fermentation is used as it increases the alcohol production by 10-12 fold as compare to batch fermentation. Large fermenter of size 125,000 gallon are used . Initially aeration is required for good growth of micro organism. Later anaerobic conditions are created by withdrawal of oxygen . It take 2-3 days for the fermentation to complete. Fermentation of sugar by Saccharomyces cervisiae is carried out for production of ethanol in a batch experiment . When the temperature was increased to 45°C, the system still showed high cell growth and ethanol production rates, while it was inhibited at 50°C. The maximum specific growth rate and the maximum specific ethanol production rate were observed between 30-45°C. pH 4.0-5.0 was the optimal range for the ethanol production process. The highest specific ethanol production rate for all the batch experiments was achieved at pH 5.0. Formation of acetic acid was increased when the pH was below 4.0, while butyric acid was produced when the pH was higher than 5.0 BATCH FERMENTATION In Batch fermentation bacteria are inoculated into the bioreactor . Then under optimal condition (temp, pH etc) the bacteria go through all the growth phases (LAG, exponential and stationary). It may be necessary to add acid or alkali to maintain pH and antifoaming agents to minimize foam under optimal condition for growth. GROWTH PHASES LAG PHASE This is the initial period just after the inoculation During lag phase microorganisms adapt to the new environment (available nutrient ,pH etc) No increase in cell number, although cellular weight may slightly increase. ACCELERATION PHASE This is the transient period during which cell start growing slowly. It connect lag phase to log phase. LOG PHASE Most active growth of micro-organism and multiplication occur during log phase. Number of cells and rate of population increases doubles with each consecutive time period. Growth rate of microbes in log phase is dependent on substrate(nutrient supply) STATTIONARY PHASE Depletion (Lessening) of nutrients and accumulation of metabolic end product . Microbial growth may either completely slow down or completely stop. The number of cells produced is limited by growth factor and as a result the rate of cell growth matches the rate of cell death. DEATH PHASE Cells die at an exponential rate At last fermentation is stopped and product is collected. Then after cleaning and sterilization of fermenter, It is ready for another batch. ADVANTAGES VERSATILE- can be used for different reaction everyday. SAFE- can be properly sterilized. Little risk of infection or strain mutation. Complete conversion of substrate is possible. DISADVANTAGS High labor cost: Skilled labor is required. High proportion of Down time between batches Product Variability – The quality and quantity (to some extent ) may vary from one batch to other batch. Safety Problem- when cleaning, filling and emptying. CONTINIOUS FERMENTATION In, continuous fermentation fresh medium flows into the fermenter continuously, and a part of the medium is withdrawn from the fermenter at the same flow rate of the inlet flow.(so that working volume remain constant). Chemostat bioreactor are frequently used in industrial manufacturing of ethanol. BY changing the rate at which the medium is added to the bioreactor the specific growth rate of the microorganism can be easily controlled. ADVANTAGES Low labor cost Growth rate is higher as nutrients are continuously added to the reactor. More efficient as the fermenter operates continuously.(No down time ) DISADVANTAGE • If contamination occur huge volume of product may be lost. Step # 01: Fermentation Dissolved sugar from ground up molasses is diluted by mixing water with it. Sulfuric acid is added with diluted sugar to prevent bacterial contamination. Yeast is added with this before feeding to fermenter. Yeast provides invertase and zymase enzymes. Only sucrose of this diluted sugar is degraded by invertase enzyme, this process is referred to as hydrolysis. Glucose and fructose are obtained by hydrolysis of sucrose. The conversion of sucrose in the fermenter was defined in 90 %. Glucose and Fructose is converted into ethanol and carbon-di-oxide, where conversion rate is defined as 95%. Catalyst of this reaction is zymase. CO2 gas leaving the fermenter carries away some ethanol with it. Step # 02: CO2 washing This gaseous stream is sent to CO2 Washer which is an absorption column. Here ethanol is absorbed by wash water. The gaseous stream leaving the absorber contains negligible amount of ethanol and this stream is vented in atmosphere. The liquid stream is recycled to the fermenter. Step # 03: Ethanol Distillation (RECOVERY) The liquid stream leaving the fermenter is a very dilute ethanol solution which also contains some sucrose, glucose, fructose and sulfuric acid. This stream is sent to the Concentration Tower which is a stripping column. Superheated steam at atmospheric pressure is used as the stripping gas (Stripping is a physical separation process by which one or more components are removed from a liquid stream by a vapor stream.) The Liquid Stream from the bottom of this tower contains water with trace of sucrose, glucose fructose and sulfuric acid. A side stream is drawn from the 6th theoretical stage of this tower, which is sent to Rectifier. The gaseous stream emerging from top of the tower is sent to Light Purification Tower. Light Purification Tower used in this process is modeled as refluxed absorber (i.e., non-heating fractional distillation by ultrasonic irradiation). Partial condenser is used in this tower. Ethanol content of the gas stream leaving the condenser of this tower is low and its flow rate is also kept very low. By this stream rest of the CO2 gas is vented to atmosphere, which was not removed by the CO2 Washer. The liquid stream leaving the bottom of this tower is a dilute ethanol solution with no other component. This is also fed to the Rectifier. The condensate collected from this unit is the light ethanol (74.23%) which is cooled to 25°C. Rectifier used in this simulation is a distillation column which has to feed, one is from Concentration Tower and other one is from Light Purification Tower. Concentrated ethanol (88.14%) is drawn out from this tower which is also cooled to 25°C. Conventional distillation/rectification systems can produce ethanol at 92-95% purity. The residual water and corn solids that remain after the distillation called “stillage.” is then centrifuged to separate the liquid (thin stillage) from the solid fragments (wet cake or distillers’ grains). The thin stillage is passed through evaporators to remove a significant portion of the water to produce thickened syrup. Usually, the syrup is blended with the distillers’ grains and dried to produce an animal feed called “distillers’ dried grains with solubles” (DDGS). Stillage, a liquid waste remaining after ethanol distillation, was collected and characterized REFERENCES Handbook of Industrial Chemistry, Volume 2, K.H. Davis, F.S. Berner. Sen, N. P., S. W. Seaman, B. P. -Y. Lau, D. Weber, and D. Lewis. 1995. Determination and occurrence of various tetrahydro-β-carboline-3carboxylic acids and the corresponding N-nitroso compounds in foods and alcoholic beverages. Food Chemistry. 54(3): 327- 337. Sree, N. K., M. Sridhar, L. V. Rao, and A. Pandey. 1999. Ethanol production in solid substrate fermentation using thermotolerant yeast. Process Biochemistry. 34: 115-119. INTERACTION WITH STUDENTS NOTE: Ask question if u have any queries about today’s lecture on whatsapp and discussion forum on LMS to describe how your personal bias might affect your interpretation of material presented today. An interactive session will also be conducted on zoom meeting regarding queries about this lecture.