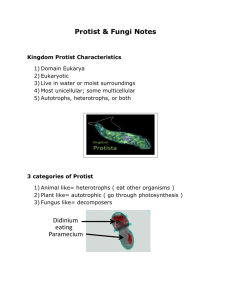
Laboratory Notes Activities 11-16 Activity No. 11: DISEASE CAUSING BACTERIA A. STAPHYLOCOCCUS The genus Staphylococcus is comprised of both pathogenic and non-pathogenic organisms. They are gram positive cocci that occur most commonly as irregular clusters of spherical cells. They are mesophilic, non-spore formers. However, they thrive in organic matter such as blood, pus and tissue fluids. • The three major’s species include: Staphylococcus aureus Staphylococcus saprophyticus Staphylococcus epidermides Infections are primarily associated with Staphylococcus aureus, pathogenic strains that are responsible for pus, other infections staphylococcal enteritis due to enteroxin contamination of food. B. SALMONELLA TYPI Salmonella typhi has also been called Ebertholla or the typhoid fever bacillus. It develops chiefly in the human body but can survive in the outside world in water and foods which are polluted by the excreta of typhoid patients or carriers. They are gram negative, straight rods, nonspore forming, facultative aerobes, motile and peritrichous. They can resist cold but are easily killed by heat and disinfectant. Activity No.12: STERILIZATION 1. Sterilization - is the process of eliminating living microorganisms using physical and chemical microbiological procedures. 2. Disinfectants - are antimicrobial agents that are applied to non-living objects to destroy microorganisms, the process of which is known as disinfection. Disinfectants should generally be distinguished from antibiotics that destroy microorganisms within the body. 3. Antiseptics - which destroy microorganisms on living tissue. 4. Sanitizers - are substances that reduce the number of microorganisms to a safe level. One official and legal version states that a sanitizer must be capable of killing 99.999%, known as a 5 log reduction, of a specific bacterial test population, and to do so within 30 seconds. The main difference between a sanitizer and a disinfectant is that at a specified use dilution, the disinfectant must have a higher kill capability for pathogenic bacteria compared to that of a sanitizer. PHYSICAL STERILIZERS 1. Heat – can be dry heat or moist heat. Heat sterilization is vital in hospital instruments where contaminated materials must be sterilized so that they be used again; and for surgical procedures At home, boiling the utensils is used to kill the microorganisms. Brief exposure of milk to heat will kill the microorganisms in milk. A. Dry Heat – kills by oxidation glassware or other item requires higher temperature like 170 0C for 2 hours of heating inside the oven. this requires longer period of heating than moist heat incineration is an example of dry heat that effectively sterilize and dispose contaminated cups, bags and dressings B. Moist Heat – is more effective that dry heat in killing microorganisms for it disrupts the hydrogen bonds that hold the proteins in their threedimensional structure steam sterilization for 15 minutes requires 121 0C to effectively eliminate the microorganisms including the endospores autoclave is an example of moist heat that uses steam under pressure the materials to be sterilized are the instruments, wire loop, culture media, etc 2. Low Temperatures – this limits the production or multiplication of microorganisms, refrigeration and freezing are used to inhibit the microbial growth most of the pathogenic microbes are unable to grow at refrigerator temperature of 5 0C freezing temperature which is at -20 0C lowers the production of microbes in food therefore the shelf life of food will be longer 3. Radiation – it may cause microbial mutations, induces the formation of toxic free radicals which increases the death rates of microbes gamma radiation, x-radiation, and ultraviolet radiation are examples of radiation which kills microorganisms by disrupting their DNA CHEMICAL STERILIZERS 1. Alcohols Alcohols, usually ethanol or isopropanol, are sometimes used as a disinfectant, but more often as an antiseptic (the distinction being that alcohol tends to be used on living tissue rather than nonliving surfaces). They have wide microbicidal activity, are non corrosive, but can be a fire hazard. They also have limited residual activity due to evaporation, which results in brief contact times, and have a limited activity in the presence of organic material. Alcohols are more effective combined with purified water—70% isopropyl alcohol or 62% ethyl alcohol is more effective than 95% alcohol, because the alcohol gets inside the cell better. 2. Aldehydes Aldehydes, such as have a wide microbiocidal activity and are sporocidal and fungicidal. They are partly in Glutaraldehyde, activated by organic matter and have slight residual activity. 3. Oxidizing agents Oxidizing agents act by oxidizing the cell membrane of microorganisms, which results in a loss of structure and leads to cell lysis and death. A large number of disinfectants operate in this way. Chlorine and oxygen are strong oxidizers. •Sodium hypochlorite - common household bleach is a sodium hypochlorite solution and is used at home to disinfect drains, toilets, and other surfaces. In more dilute form, it is used in swimming pools, and in still more dilute form, it is used in drinking water. When pools and drinking water are said to be chlorinated, it is actually sodium hypochlorite or a related compound, not pure chlorine, which is being used. •Chloramine is often used in drinking water treatment instead of chlorine because it produces fewer disinfection byproducts, which can be harmful. •Chlorine dioxide is used as an advanced disinfectant for drinking water to reduce waterborne diseases. In certain parts of the world, it has largely replaced chlorine because it forms fewer byproducts. Sodium chlorite, sodium chlorate, and potassium chlorate are used as precursors for generating chlorine dioxide. •Hydrogen peroxide is used in hospitals to disinfect surfaces. It is sometimes mixed with colloidal silver. It is often preferred because it causes far fewer allergic reactions than alternative disinfectants. Also used in the food packaging industry to disinfect foil containers. A 3% solution is also used as an antiseptic. When hydrogen peroxide comes into contact with the catalase enzyme in cells it is broken down into water and a hydroxyl free radical. It is the damage caused by the oxygen free radical that kills bacteria. However, recent studies have shown hydrogen peroxide to be toxic to growing cells as well as bacteria; its use as an antiseptic is no longer recommended. •Iodine is usually dissolved in an organic solvent or as Lugol's iodine solution. It is used in the poultry industry. It is added to the birds' drinking water. Although no longer recommended because it increases scar tissue formation and increases healing time, tincture of iodine has also been used as an antiseptic for skin cuts and scrapes. •Ozone is a gas that can be added to water for sanitation. •Potassium permanganate (KMnO4) is a red crystalline powder that colors everything it touches, and is used to disinfect aquariums. It is also used widely in community swimming pools to disinfect ones feet before entering the pool. Typically, a large shallow basin of KMnO4 water solution is kept near the pool ladder. Participants are required to step in the basin and then go into the pool. Additionally, it is widely used to disinfect community water ponds and wells in tropical countries, as well as to disinfect the mouth before pulling out teeth. It can be applied to wounds in dilute solution; potassium permanganate is a very useful disinfectant. 4. Phenolics Phenolics are active ingredients in some household disinfectants. They are also found in some mouthwashes and in disinfectant soap and hand washes. Phenol is probably the oldest known disinfectant as it was first used by Lister, when it was called carbolic acid. It is rather corrosive to the skin and sometimes toxic to sensitive people. O-phenylphenol is often used instead of Phenol, since it is somewhat less corrosive. Hexachlorophene is a phenolic that was once used as a germicidal additive to some household products but was banned due to suspected harmful effects. 5. Quaternary ammonium compounds Quaternary ammonium compounds (Quats), such as benzalkonium chloride, are a large group of related compounds. Some have been used as low level disinfectants. They are effective against bacteria, but not against some species of Pseudomonas bacteria or bacterial spores. Quats are biocides which also kill algae and are used as an additive in large-scale industrial water systems to minimize undesired biological growth. Quaternary ammonium compounds can also be effective disinfectants against enveloped viruses. 6. Acids Organic acids can control microbial growth and are frequently used as preservatives. Sorbic, benzoic, lactic, and propionic acids are used to preserve foods and pharmaceuticals. Benzoic, salicylic, and undecylenic acids are used to contol fungi that cause diseases such as athlete’s foot. 7. Heavy Metals Heavy metals used in disinfectant and antiseptic formulations, examples- silver, copper, mercury and zinc have antimicrobial properties. Silver nitrate is used to prevent gonococcal eye infections Mercurochrome and merthiolate are applied on the skin after minor wounds Zinc is used as antifungal antiseptics Copper sulfate is used as an algicide Activity No. 12: PHYSICAL AGENTS OF STERILIZATION PHYSICAL AGENTS A. HEAT 1.Moist Heat Sterilization Boiling Steam under Pressure Pasteurization 2. Dry Heat Sterilization Direct Flaming Incineration Hot Air Sterilization B. FILTRATION C. LOW TEMPERATURE 1. Low Temperature 2. Deep Freezing D. DESSICATION/DEHYDRATION E. OSMOTIC PRESSURE/PLASMOLYSIS F. RADIATION MECHANISM OF ACTION FUNGI Fungi are spore-producing organisms; a single-celled or multicellular organism without chlorophyll that reproduces by spores and lives by absorbing nutrients from organic matter. They are classified as eukaryotes, i.e., they have a diploid number of chromosomes and a nuclear membrane and have sterols in their plasma membrane. Genetic complexity allows morphologic complexity and thus these organisms have complex structural features that are used in speciation. Fungi are neither plant nor animal, but have some characteristics of each. They cannot move about like an animal, do consume organic matter, have no chlorophyll as do plants, and cannot manufacture their own energy. They have a true nucleus in their cells and are able to sexually reproduce by combining like strains of nucleus. They can also reproduce by spores similar to some of the more primitive plants e.g. Ferns, Liverworts and Mosses. Modern molecular studies have shown that fungi are more closely related to animals than to plants. The structures of fungi are microscopic and not visible to the naked eye. Some are unicellular like yeast, but most string their cells together in long, thread-like strands called hypha. Most fungi produce an extensive system of hyphae, which may be visible when growing thickly in a mass called mycelium (commonly referred to as mold). Mycelium can be of any size from tiny clusters to massive acre wide systems, which effectively form the feeding and growing body of the fungus. Fungi are divided into two groups: 1. 2. Microscopic fungi a) molds b) Yeasts Macroscopic fungi a) mushroom b) puffballs c) gill fungi YEASTS are eukaryotic microorganisms with round to oval shape; with about 1,500 species currently described; they dominate fungal diversity in the oceans. Most reproduce asexually by budding, although a few do so by binary fission. Yeasts are unicellular, although some species with yeast forms may become multicellular through the formation of a string of connected budding cells known as pseudohyphae, or false hyphae as seen in most molds. Yeast size can vary greatly depending on the species, typically measuring 3–4 µm in diameter, although some yeasts can reach over 40 µm. The yeast species Saccharomyces cerevisiae has been used in baking and fermenting alcoholic beverages for thousands of years. Other species of yeast, such as Candida albicans, are opportunistic pathogens and can cause infection in humans. Growth and nutrition Yeasts are chemoorganotrophs as they use organic compounds as a source of energy and do not require sunlight to grow. The main source of carbon is obtained by hexose sugars such as glucose and fructose, or disaccharides such as sucrose and maltose. Some species can metabolize pentose sugars like ribose, alcohols, and organic acids. Yeast species either require oxygen for aerobic cellular respiration (obligate aerobes), or are anaerobic but also have aerobic methods of energy production (facultative anaerobes). Unlike bacteria, there are no known yeast species that grow only anaerobically (obligate anaerobes). Yeasts grow best in a neutral or slightly acidic pH environment. Yeasts will grow over a temperature range of 10°-37°C (50°98.6°F), with an optimal temperature range of 30°-37°C (86°98.6°F), depending on the type of species (S. cerevisiae works best at about 30°C(86°F)). Above 37°C (98.6°F) yeast cells become stressed and will not divide properly. Most yeast cells die above 50°C (122°F). If the solution reaches 105°C (221°F) the yeast will disintegrate. There is little activity in the range of 0°10°C (32°-50°F). The cells can survive freezing under certain conditions, with viability decreasing over time. Activity No. 13: MOLDS MOLDS include all species of microscopic fungi that grow in the form of multicellular filaments, called hyphae. In contrast, microscopic fungi that grow as single cells are called yeasts. A connected network of these tubular branching hyphae has multiple, genetically identical nuclei and is considered a single organism, referred to as a colony or in more technical terms a mycelium. Molds do not form a specific taxonomic or phylogenetic grouping, but can be found in the divisions Zygomycota, Deuteromycota and Ascomycota. Although some molds cause disease or food spoilage, others are useful for their role in biodegradation or in the production of various foods, beverages, antibiotics and enzymes. Although molds grow on dead organic matter everywhere in nature, their presence is only visible to the unaided eye when mold colonies grow. A mold colony does not comprise discrete organisms, but an interconnected network of hyphae called a mycelium. Nutrients and in some cases organelles may be transported throughout the mycelium. In artificial environments like buildings, humidity and temperature are often stable enough to foster the growth of mold colonies, commonly seen as a downy or furry coating growing on food or other surfaces. Some molds can begin growing at temperatures as low as 2°C. When conditions do not enable growth, molds may remain alive in a dormant state depending on the species, within a large range of temperatures before they die. Common molds Acremonium Aspergillus Cladosporium Fusarium Mucor Penicillium Rhizopus Stachybotrys Trichoderma Activity No. 14: FACTORS THAT AFFECT YEAST GROWTH Yeasts are microscopic organisms that exist naturally on the surface of the earth. They are noted for their ability to ferment carbohydrates to produce various food products, including bread, beer, wine, and cheese. During fermentation, yeast cells break down complex sugars into simple sugars, which are further hydrolyzed into CO2 and ethyl alcohol (ethanol). Yeast growth is affected by several, including temperature, pH, and nutrient content. There are several factors which affect the growth of yeast. Yeast has been used in the fermentation of foods like bread, wine, and beer. The by-products of the fermentation process are carbon dioxide and ethanol. Activity No. 15: VIRUS: THE MODEL COVID-19 is the disease caused by SARS-CoV-2, the coronavirus that emerged in December 2019. COVID-19 can be severe, and has caused millions of deaths around the world as well as lasting health problems in some who have survived the illness. The coronavirus can be spread from person to person The coronavirus particles are organized with long RNA polymers tightly packed into the center of the particle, and surrounded by a protective capsid, which is a lattice of repeated protein molecules referred to as coat or capsid proteins. In coronavirus, these proteins are called nucleocapsid (N) Activity No. 16: PARASITOLOGY Organism 1. Escherichia coli Description Escherichia coli (E. coli) is a Gram-negative, rodshaped, facultative anaerobic bacterium. This microorganism was first described by Theodor Escherich in 1885. The non-parasitic form is excreted via stools and can live as commensals in the large intestine. 2. Entamoeba coli 3. Entamoeba histolytica 4. Balantidium coli 5. Ascaris lumbricoides/Roundworm/Adult 6. Enterobius vermicularis/Pinworm/Adult 7. Necator americanus/Hookworm/Adult 8. Adult Flatworm/Tapeworms (Scolex and Proglottid) T saginata “Beef Tapeworm” T solium “Pork Tapeworm” Classification (R Whittaker) Monera Unicellular Prokaryotic Cell