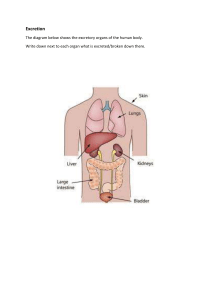
Drug Approval / Regulation Pure food and Drug Act 1906—established government control of labeling medicines. Food Drug and Cosmetic Act 1983 and amendments o Thorough testing of drugs o Proof of safety and efficacy of drugs FDA must approve a drug before it may be marketed o Black Box Warnings—created to regulate drugs with serious problems with potential for causing serious injury or death o FOUR STAGES OF APPROVAL: 1. Preclinical Investigation: o o o o o NO HUMANS Lab research Only on cells and animals Determines drug-dose range Examines adverse side effects Inconclusive since cannot be tested on humans 2. Clinical Investigation o In 3 different stages—clinical phase trials o Longest part o Evaluates human benefits ✓ HUMAN TRIALS BEGIN o Tests healthy humans first • Then on those with ailments 3. Review of New Drug Application (NDA) o Applied if clinical investigations are positive—even if precautions are o o o o noted Avg 17-24months Drug approved—processed continued Drug rejected—suspended Investigational New Drug Application (IND) • Phase I clinical trials if there are significant therapeutic benefits 4. Post-marketing Surveillance o Placed on market o Surveyed for harmful effects in general population o Annual public meetings • Will withdraw if serious problems found FDA Emergency Use Authorization (EUA) o Used during public health emergencies (Covid-19 vaccines) o Allows use of unapproved products to prevent life-threatening diseases/conditions with no alternatives o Potential benefits outweigh the risks o Goal—more timely access for medical treatment Controlled substances: o Drugs with significant potential for abuse are placed into five categories called schedules o Schedule I (highest potential) Schedule V (lowest potential) o US DRUG SCHEDULES BELOW DRUG SCHEDULE Abuse Potential Potential for PHYSICAL Potential for PHYSIOLOGICA Therapeutic Use I Highest dependence High L dependence High II High High High III Moderate Moderate High IV Lower Lower Lower V Lower Lower lower Limited or none Limited or none Used with prescription Used with prescription Used without prescription PRINCIPALS OF PHARMACOLOGY ACROSS THE LIFESPAN Pharmacokinetics o Effects of the BODY on the drug o Study of drugs movement through the body o How the body handles the medications o Understand/predicts actions and side effects o Understands obstacles to target Drugs in the Body – The greatest barrier for a drug is CROSSING MEMBRANES – hence drugs administer intravenously are most effective as they do not have any membrane barrier to cross Enteral drugs are broken down by the stomach acids and digestive enzymes Organs attempt to excrete med Phagocytes may attempt to remove as they are seen as foreign objects ADME ABSORPTION – HOW WILL IT GET IN? o Movement of drug from site of admission to the bloodstream o This is the PRIMARY PHARMOKINETIC FACTOR—determining the length of time it will take to produce an effect o Factors affecting Formulation (enteric-coated pills may take longer for example) Dose Route Size of drug molecule—smaller the easier Surface area of absorption site—larger more difficult Digestive motility Blood flow – ↑profusion is favorable Lipid soluability—these stay in our body longer Water-soluble drugs excrete faster Degree of ionization Lipid soluble Nonionized easily absorbed by cell membranes DO WELL IN ACIDIC Water soluble ionized not as easily absorbed DO WELL IN ALKALINE pH of local environment drug-drug and food-drug interactions dietary/herbal product-drug interaction Distribution – TRANSPORT/ WHERE WILL IT GO? o TRANSPORT of the drug throughout the body/ movement into various tissues o Process by which drug molecules will leave blood stream and are transported by bodily fluids to site of action o Simplest factor determining distribution is the amount of blood flow to body tissues, also solubility of protein binding site. REMEMBER ↑profusion is always our friend. o Physical properties of drug have high influence o DRUGS BIND WITH PLASMA PROTEINS: o Drug can remain free or bind to plasma protein ( ie albumin) o Many drugs form a drug-protein complex—binding and never reaching target cells Protein-binding may affect drug activity in one of two ways: either by changing the effective concentration of the drug at its site of action or by changing the rate at which the drug is eliminated, thus affecting the length of time for which effective concentrations are maintained. Why is it important for the nurse to consider protein binding when giving drugs? This is important because it is the free drug that traverses cell membranes and produces the desired effect. It is also important because a protein-bound drug can act as a reservoir that releases the drug slowly and thus prolongs its action. Two drugs can compete for plasma-protein binding sites The drug with a higher affinity (likeness) will gain the site and displace the other Displaced drug can reach high levels & can produce adverse/toxic effects When two, highly protein-bound drugs (A and B) are added into the same biological system it will lead to an initial small increase in the concentration of free drug A (as drug B ejects some of the drug A from its proteins). o Blood-brain barrier and fetal-placenta barrier Anatomic barrier that prevents many chemicals and medications from entering Makes brain tumors difficult to treat Fetal-placental barrier protects the fetus METABOLISM- HOW IS IT BROKEN DOWN? o o o o Biotransformation Chemically converts drug so it is easily excreted Liver is the primary site of metabolism Side chains—conjugates, makes drug more water soluble to be easily excreted by kidneys o METABOLISM IN THE LIVER Accomplished by the Hepatic microsomal enzyme system Primary action—to inactivate drugs and accelerate excretion Pro-drugs have no pharmacological activity unless first metabolized to their active form by the body Changes in the function of the hepatic microsomal enzymes can signifigantly affect drug metabolism REMEMBER: Children immature liver | Older population deteriorating liver | Hepatic failure med admin gets complicated o Dosages should be reduced in patients with impaired metabolic activity to prevent toxicity Some drugs increase metabolic activity in the liver (enzyme induction) o Enzyme induction could increase the metabolism and clearance of a pharmacologically active drug leading to a reduction in pharmacological activity Some drugs reduce metabolic activity in the liver (enzyme inhibitors) o Enzyme inhibition reduces metabolism In general, the metabolism of a drug decreases its therapeutic effect FIRST-PASS EFFECT—The first pass effect is a phenomenon in which a drug gets metabolized at a specific location in the body that results in a reduced concentration of the active drug upon reaching its site of action or the systemic circulation. EXCRETION- HOW DOES IT LEAVE YOUR BODY? o Site of excretion KIDNEYS o Easily excreted – easily filtered (glomerulus) Free drugs remember those protein-binded drugs are locked up (NOT FREE) therefore, they can be released late, and they cause ↑risk of toxicity – those sneaky little criminals! Perhaps they will be overrun by a sneakier criminal for that binding sites.. hint hint! if you don’t get it go back up and review this!!! Water soluble agents think waters free movement Electrolytes Small molecules o Secreted into distal tubule of nephron Drug protein complexes Large substances o Secretion mechanism is LESS ACTIVE in infants and older adults o pH of filtrate can INCREASE excretion o FACTORS AFFECTING DRUG EXCRETION Liver/kidney impairment Blood flow remember a good flow is our FRIEND Degree of ionization of drug REMEMBER: ionized = water soluble and WE KNOW water soluble is EASILY EXCRETED (DOSING CONSDERATION HERE) Lipid solubility of drug The key point to remember about metabolism is that it makes drugs less lipid soluble, more water soluble, and therefore easier to excrete from the body Drug protein-complexes Metabolic activity the goal is to make the drug easier to excrete you need good hepatic function to do so SO REMEMBER TO KEEP SPECIAL CONSIDERATION WHEN DELIVERING MEDS TO: infants, older adults, and those with hepatic failure! Acidity or alkalinity (pH) In acidic urine, alkaline drugs are more readily ionized. Ionized substances are more soluble in water so dissolve in the body fluids more readily for excretion. Respiratory, glandular, or biliary activity Drugs are also excreted via lungs, sweat glands, skin, intestinal tract and breastmilk—always consider this Biliary excretion involves active secretion of drug molecules or their metabolites from hepatocytes into the bile. The bile then transports the drugs to the gut, where the drugs are excreted through feces—sounds like you may want a functioning liver for this form of excretion (CONSIDERATION!) o RENAL FAILURE DIMINISHES EXCRETION Drugs are retained for extended periods Dosages must be reduced LET’S TALK DIALYSIS—it’s an ARTIFICIAL KIDNEY Rapidly flushes you out Nursing consideration would be to HOLD meds until after dialysis to preserve a therapeutic effect o ENTEROHEPATIC RECIRCULATION Enterohepatic recirculation is the process by which biliary excreted drug is reabsorbed in the intestine instead of being removed from the body. This can result in a lagging secondary absorption process and increases in drug exposure. Pertains to IV administration DRUG PLASMA CONCENTRATION & THERAPEUTIC RESPONSE Concentration of med in target tissue is not easy to measure so it must be measured in plasma: o Minimum effective concentration—amt required to produce therapeutic effect o Toxic concentration—level that would result in serious adverse effects o Therapeutic ranges—concentration between minimum effective concentration and toxic concentration Onset of drug action—time takes to produce therapeutic event Peak plasma level—when med reaches its highest concentration in blood Duration of drug action—amt of time drug maintains a therapeutic effect Plasma Half-Life (t½) – USED FOR Dosing & Frequency Time takes for drug to decrease by 50% Metabolism/excretions determines Greater the half-life, the longer it takes to excrete Doesn’t change with dose How does a drug reach and maintain its therapeutic effect? Repeated doses Drug accumulates in blood Plateau is reached Amount administered = amount excreted STEADY STATE— Amount administered = amount excreted A stable/consistent level Takes approximately 5 half-lives to reach LOADING DOSE Single large dose used to raise plasma concentration to a therapeutic level Higher amt given Plateau reached faster MAINTENANCE DOSE - Keeps plasma concentration @ therapeutic level SERUM DRUG LEVELS: Peak level—highest plasma concentration Trough level—drug at its lowest level—30 min before next dose given Therapeutic range—results show if patient has correct/therapeutic amt of drug FIVE RIGHTS OF ADMINISTRATION: 1. Right person 2. Right med THREE CHECKS—checks with MAR when 1. Removing from drawer 2. When preparing med 3. Right before administering 3. Right dose 4. Right Route 5. Right time ALWAYS CHECK ALLERGIES BEFORE Pharmacodynamics Frequency Distribution Curve How a medication changes the body Helps predict the change in a person Helps determine safe/effective treatment MEDIAN EFFECTIVE DOSE o Middle of curve o Produces therapeutic effect in 50% of a group o Called average or standard dose o Many pts require less or more MEDIAN LETHAL DOSE o Asses safety o Shown on curve o Determined in preclinical trials o In 50% of group of animals o NOT ON HUMANS THERAPEUTIC INDEX o Measure of drugs safety margin o THE HIGHER THE VALUE – SAFER THE DRUG o TI = Median lethal dose Median effective dose MEDIAN TOXICITY LEVEL o Toxicity in 50% of group From animal data Adverse effects from clinical trials o THIS IS NEEDED BECAUSE LETHAL DOSES CAN NOT BE TESTED ON HUMANS TWO WAYS TO COMPARE MEDICATIONS: 1. Potency – a higher potency produces a therapeutic effect with a smaller dose 2. Efficacy – capacity to produce an effect – efficacy is always more important than potency RECEPTOR SITE: The better the fit of a receptor site and a drug molecule = better response Intrinsic activity Ability to activate the receptor Affinity – likeness o Strength of a drugs binding ability o High affinity = strongly attracted to receptor sites, usually potent drugs o Low affinity = less attracted, weaker drugs Types of Drug-Receptor Interactions: Agonist—drug molecule attaches to receptor o Promotes a function/promotes the same type of response as endogenous substance o Sometimes produces greater maximal response o Affinity and intrinsic acidity Partial agonist—medication that produces a weaker response than agonist Antagonist o Affinity for receptor o No intrinsic activity o Prevents/blocks a response from happening ADVERSE EVENT (AE)—Unintended Anaphylaxis—severe massive histamine response, you can see: • Hypotension • Tachycardia • Dyspnea Teratogenic effects—causes harm to fetus Idiosyncratic response—an unexplained drug reaction Carcinogenic—potential cause of cancer TOXICITIES Neurotoxicity (CNS) • Drowsiness • Auditory/visual disturbances • Seizures Hepatotoxicity (liver) • Drug will not be metabolized efficiently Nephrotoxicity (kidneys) • Decreased urine output • ↑ blood urea nitrogen • ↑ serum creatinine • Altered acid-base balance • Electrolyte imbalance Ototoxicity (8th cranial nerve) • Tinnitus • Hearing loss • Light-headed • Vertigo • Nausea Cardiotoxicity (heart) • Irregularities in conduction • HF • Damage to myocardium Immunotoxicity (immune system) Immunosuppression PATIENT Infant(birth-12m) RELATED VARIABLE Primary goal—administer full dose without spitting out Held and cuddled Allow to swallow before administering more w/ syringe Insert into buccal cavity Toddler(1-3y) Preschool(3-5y) School-aged(5-12y) Adolescent(13-16y) Young adulthood18-40y) Middle aged adults Older adults(65+) Immature hepatic function alters metabolism Never give IM into gluteal—causes permanent damage to sciatic nerve Always putting things in mouth, major med safety concern at home Parent education—lock meds up Flavored elixir’s never tell a child meds are candy May need help restraining for IV or injections May start to understand the need Safe storage of meds Can begin to help with administration Briefly explain that the med will help them feel better Mixing with food/beverage After walking you may use ventrogluteal as IM site More detailed explanation is reasonable Let them choose a drink they like Make them feel like a participant in the healthcare team Independence leads to self-administration of meds Parents should be educated and aware of recreational use Most common: for skin, headaches, contraceptives, alcohol/tobacco, sport related injuries Privacy is key, talk to them like an adult Often embarrassed in front of peers which can impact taking medications at school Need support, approval, and presence Educate about: Hazards of tobacco and substance abuse, Sexual intercourse, Eating disorders Generally good health ADME at their PEAK Medical adherence and compliance is usually good 18-24 there is concern for illicit drug use Health remains the same as young adult until 45y Numerous transitions occur Begin to look for: hypotension, obesity, arthritis, cancer, and anxiety Diabetic screening begins Age related change—response to medications Absorption—decreased gastric motility/increased pH Distribution—aging liver produces less albumin (protein-binding) which increased higher level of free drugs Metabolism—again liver results in first-pass metabolism MATERNITY CHANGED IN PREGNANCY ABSORPTION Hormonal changes affect absorption Inhaled drugs may be absorbed faster Teratogen A substance, organism, or physical agent to which an embryo or fetus is exposed that causes permanent abnormality in structure or function and causes retardation or death No absolute teratogens: risk increases with dose Current FDA Pregnancy Category Category A Interpretation Drug Adequate, well-controlled studies in pregnant women Have not shown an increased risk of fetal abnormalities to the fetus in any trimester of pregnancy Animal studies have revealed no evidence of harm to the fetus; however, there are no adequate and wellcontrolled studies in pregnant women. Animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate risk to the fetus in any trimester. Prenatal multivitamins, insulin, thyroxine, folic acid C Animal studies have shown an adverse effect and there are no adequate and well-controlled studies in pregnant women. OR No animal studies have been conducted and there are no adequate and well-controlled studies in pregnant women. Most prescription medicines; antimicrobials such as clarithromycin, fluoroquinolones, and Bactrim; selective serotonin reuptake inhibitors (SSRIs); corticosteroids; and most antihypertensives D Adequate well-controlled or observational studies in pregnant women have demonstrated a risk to the fetus. However, the benefits of therapy may outweigh the potential risk. Alcohol, ACE inhibitors, angiotensin receptor blockers (ARBs) in the second and third trimesters, gentamicin, carbamazepine, cyclophosphamide, lithium carbonate, methimazole, mitomycin, nicotine, nonsteroidal anti-inflammatory drugs (NSAIDs) in the third trimester, phenytoin, propylthiouracil, streptomycin, tetracyclines, valproic acid X Adequate well-controlled or observational studies in animals or pregnant women have demonstrated positive evidence of fetal abnormalities or risks. The use of the product is contraindicated in women who are or may become pregnant. There is no indication for use in pregnancy B Penicillin, cephalosporins, azithromycin, acetaminophen, ibuprofen in the first and second trimesters Clomiphene, fluorouracil, isotretinoin, leuprolide, menotropins, methotrexate, misoprostol, nafarelin, oral contraceptives, raloxifene, ribavirin, statins, temazepam, testosterone and thalidomide, and warfarin Drugs Secreted into Breast Milk Fortunately, few instances of harm to infant Dangerous drugs usually have safe alternatives Drugs with high protein-binding ability are less likely to enter breast milk Mother should check all medications with OB MD during lactation Factors That Affect Drug Exposure Through Lactation Time between drug administration and breast feeding Mother's use of illicit drugs Amount of drug administered Amount that reaches fetus tissue Infant's ability to metabolize drug