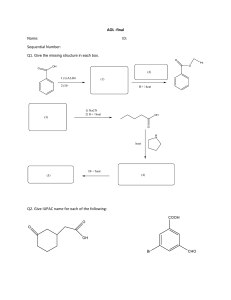
ORGANIC CHEMISTRY NOTES & Past Papers AS- REVISION NAME:_________________________________________ Zafar Iqbal Ch. ALKANE ALKENE ALCOHOL ALDEHYDE KETONE Carboxylic Acid Pg-1 complete incomplete combustion Alkanes Free radical substitution Sunlight x2 x2 x2 x2 Zafar Iqbal Ch. Pg-2 Preparation of Alkene 1- By the ________________________________of Alkane 2- By the ______________________________________of Haloalkane 3- By the ______________________________________of Alcohol Spider diagram of Alkene Addition Polymerization Cold dilute KMnO 4/H+ HX /heat Room temp. Alkene X2/CCl4 Room temp. Dark Aq.Bromine Hot & conc.KMnO 4/H+ H2 Ni/Pt 180 oC Excess Combustion. H2O(g) 300oC + H3PO4 60-70 atm Limited O2 O2 H2O(l)/H2SO4 ZAFA R IQ BAL CH. Pg-3 1. hot concentrated MnO4– / H+ CH3CH CHCH3 Cl 2 cold dilute MnO4– / H+ A B C K2Cr2O7 / H + ZAFA R IQ BAL CH. D [4] Pg-4 2. Crude oil is the principal source of hydrocarbons. The following are examples of such hydrocarbons. H H H C C H H H H H ethane C C CH3 H propene cyclohexene (a) Give the structural formulae of the organic products in the following reactions. (i) The reaction of ethane with bromine in the presence of u.v. light. ZAFA R IQ BAL CH. (ii) The polymerisation of propene. (iii) The oxidation of propene with cold, acidified potassium manganate(VII). (iv) The reaction of cyclohexene with hydrogen bromide. (v) The reaction of cyclohexene with hot acidified potassium manganate(VII). [5] Pg-5 HX Sunlight HX Room temp. PX3 ,PX 5 , SOX 2 X2 NaOH/KOH Ethanol (heat + reflux) Limited NH3 ethanolic Haloalkane ZAFA R-X NaOH/KOH Aqueous/Heat + Refulux KCN/NaCN R IQ BAL CH. Heat + reflux LiAlH4 in dry ether or H 2 (Pt/Ni) Reduction R-X Hydrolysis H3O+ R-X OH- Pg-6 3. Complete the following reaction scheme which starts with propene. In each empty box, write the structural formula of the organic compound that would be formed. Br2 CH3CH=CH2 KMnO4 /H+ cold, dilute A B ZAFA R IQ BAL CH. D HBr KCN in NH3 aqueous ethanol in an excess C NaOH(in ethanol) heat under reflux H2SO4(aq) heat under reflux E F G [7] Pg-7 Hydroxy Compounds. Distilled off o P alcohol HEAT+ REFLUX Soalcohol oxidation Toalcohol PX3,PX5,SOX2 Chemical Properties of alcohol. HX Reactive metal 170oC Conc.H2SO4 Complete Combustion Carboxylic acid ZAFA R IQ BAL CH. Pg-8 4. Crotyl alcohol, CH3CH=CHCH2OH, is a colourless liquid which is used as a solvent. In the boxes below, write the structural formula of the organic compound formed when crotyl alcohol is reacted separately with each reagent under suitable conditions. If you think no reaction occurs, write 'NO REACTION' in the box. A Br2 in an inert organic solvent B PCl 5 C H2 and Ni catalyst D NaBH4 E K2Cr2O7 / H+ heat under reflux [5] ZAFA R IQ BAL CH. Pg-9 5. A series of reactions based on propan-1-ol is shown. CH3CH2CH2OH reaction 1 steam CH3CH=CH2 reaction 2 catalyst reaction 3 U (C3H8O) HBr V CH3CH2CO2H (a) Suggest a suitable reagent and conditions for reaction 1. .............................................................................................................................................. [2] (b) (i) Write an equation for reaction 2, using [O] to represent the oxidising agent. ....................................................................................................................................... [1] (ii) Suggest a suitable reagent and conditions for reaction 2. ....................................................................................................................................... [2] (c) Give the structural formulae of U and V. U ................................................................................................................................................ V ................................................................................................................................................ [2] (d) Suggest a suitable reagent and conditions for reaction 3. .................................................................................................................................................... .............................................................................................................................................. [2] [Total: 9] ZAFA R IQ BAL CH. Pg-10 6. Compounds containing the allyl group, CH2=CHCH2–, have pungent smells and are found in onions and garlic. Allyl alcohol, CH2=CHCH2OH, is a colourless liquid which is soluble in water. (a) Allyl alcohol behaves as a primary alcohol and as an alkene. Give the structural formula of the organic compound formed when allyl alcohol is reacted separately with each of the following reagents. (i) acidified potassium dichromate(VI), heating under reflux (ii) bromine in an inert organic solvent (iii) cold, dilute, acidified potassium manganate(VII) (iv) hot, concentrated, acidified potassium manganate(VII) [5] ZAFA R IQ BAL CH. Pg-11 7. Food additives are substances added to food to preserve the flavour or to improve its taste and appearance. European Union legislation requires most additives used in foods to be labelled clearly in the list of ingredients, either by name or by an ‘E number’. E296 is malic acid which occurs in unripe fruit. Malic acid has the structural formula HO2CCH2CH(OH)CO2H. (a) Some reactions of malic acid are shown below. In the boxes below, give the structural formulae of organic compounds A to F. heat with CH3OH / H+ HO2CCH2CH(OH)CO2H heat with CH3CO2H / H+ concentrated H2SO4 A B C hot concentrated MnO4– / H+ steam / H3PO4 cold dilute MnO4– / H+ D E F [6] ZAFA R IQ BAL CH. Pg-12 Carbonyl Compounds. ALDEHYDE Oxidation Reduction Chemical Properties of Aldehyde HCN - reduction H3O+ OH ZAFA R IQ BAL CH. Pg-13 8. hot concentrated MnO4– / H+ CH3CH CHCH3 Cl 2 cold dilute MnO4– / H+ A B C K2Cr2O7 / H+ D ZAFA [4] R IQ BAL CH. 9. Lactic acid may be synthesised from ethanol by the following route. CH3CH2OH step 1 CH3CHO step 2 CH3CH(OH)CN step 3 CH3CH(OH)CO2H Give the reagent(s) and essential condition(s) for each step. reagent(s) condition(s) step 1 step 2 step 3 [6] Pg-15 Carbonyl Compounds. KETONE Reduction HCN OH- H3O+ ZAFA R IQ BAL CH. reduction Chemical Properties of Ketones Pg-16 10. Complete the following reaction scheme which starts with propanone. In each empty box, write the structural formula of the organic compound that would be formed. NaBH4 CH3COCH3 HCN V PCl5 W X ZAFA R IQ BAL CH. dil H2SO4 heat Y conc. H2SO4 170 °C Br2 Z [6] Pg-17 11. Some reactions involving 2-bromopropane are shown. CH3CHBrCH3 2-bromopropane reaction 1 CH3CH(NH2)CH3 reaction 2 X reaction 3 hydrolysis reaction 6 HBr Y reaction 5 CH3CH2CH2OH reaction 4 CH3CH2CO2H (and CH3CH(NH2)CH3 which is removed) (i) State the reagent needed for reaction 1. ....................................................................................................................................... [1] (ii) State the reagent needed for reaction 2. ....................................................................................................................................... [1] (iii) Give the structural formula of X. [1] (iv) Name the type of reaction involved in reaction 4 and suggest a suitable reagent. ............................................................................................................................................. ....................................................................................................................................... [2] (v) State the name of a solid catalyst for reaction 5. ....................................................................................................................................... [1] ZAFA R IQ BAL CH. Pg-18 12. A reaction sequence based on propan-1-ol is shown. O CH3CH2C OH reaction 1 CH3CH CH2 reaction 3 CH3CH2CH2 reaction 4 OH CH3CH2CH2 Br propan-1-ol reaction 2 O CH3CH2C OH H reaction 5 NaCN / H+ CH3CH2C CN H (a) Reactions 1 and 2 can both be carried out using the same reagents. (i) Identify suitable reagents for reactions 1 and 2. ............................................................................................................................................. ....................................................................................................................................... [1] (ii) State and explain how the reaction should be carried out to ensure that reaction 2 rather than reaction 1 occurs. ............................................................................................................................................. ............................................................................................................................................. ............................................................................................................................................. ....................................................................................................................................... [2] (b) Identify the necessary reagents and conditions for each of reactions 3 and 4. reaction 3 ................................................................................................................................... .................................................................................................................................................... reaction 4 ................................................................................................................................... .................................................................................................................................................... [2] ZAFA R IQ BAL CH. Pg-19 13. Some reactions of compound P, C H O, are shown. 5 8 compound Q H2(g) Ni(s) catalyst compound R NaBH4 cold, acidified KMnO4(aq) O compound P compound S ZAFA R IQ BAL CH. OH–(aq) I2(aq) O compound T + – O (a) (i) Give the structures for organic compounds Q, R, S and T. Q R S T [4] Pg-20 14. 2-hydroxypropanoic acid can be synthesised in four steps from ethanoic acid. O step 1 OH O step 2 Cl O O step 3 OH CN ethanoic acid O step 4 OH OH O 2-hydroxypropanoic acid (a) (i) Suggest a reagent for step 2. ....................................................................................................................................... [1] (ii) Suggest reagents and conditions for steps 1 and 4. step 1 .................................................................................................................................. step 4 .................................................................................................................................. [2] ZAFA R IQ BAL CH. Pg-21 15. Some reactions based on 1-bromobutane, CH3(CH2)3Br, are shown. reaction 1 CH3(CH2)3OH reaction 5 CH3(CH2)2CHO CH3(CH2)3Br reaction 2 reaction 3 ZAFA CH3(CH2)3C≡N reaction 6 CH3(CH2)2COOH CH3CH2CH=CH2 R IQ BAL CH. reaction 4 CH3(CH2)3COOH (a) For each of the reactions state the reagent(s), the particular conditions required, if any, and the type of reaction. For the type of reaction choose from the list. Each type may be used once, more than once or not at all. Each reaction may be described by more than one type. reaction elimination hydrolysis substitution oxidation addition condensation reagent(s) and conditions type(s) of reaction 1 2 3 4 5 6 [6] Pg-21 16. Some reactions are shown, based on methylpropan-2-ol, (CH3)3COH. (CH3)3CBr reaction 1 reaction 2 (CH3)3COH reaction 3 (CH3)2C=CH2 reaction 4 (CH3)3CBr and (CH3)2CHCH2Br (a) For each of the reactions state the reagent(s), the particular conditions required, if any, and the type of reaction. For the type of reaction choose from the list. Each type may be used once, more than once or not at all. Each reaction may be described by one or more than one type. reaction hydrolysis dehydration substitution oxidation addition condensation reagent(s) and conditions type(s) of reaction 1 2 3 4 [5] ZAFA R IQ BAL CH. [3] Pg-22 17. The structure of glycolic acid is shown. H H O C C OH OH glycolic acid (a) Complete the table to show what you would observe when an aqueous solution of glycolic acid is added separately to each of the reagents. If a reaction occurs, state the functional group of glycolic acid that is responsible for the reaction. reagent observation with glycolic acid does a reaction occur? / functional group Na2CO3(aq) 2,4-DNPH acidified Cr2O72– [4] (b) Two reaction sequences to make glycolic acid are shown. HCN and NaCN sequence A HCHO sequence B CH3CO2H (i) reaction 1 Br2 reaction 3 X reaction 2 CH2BrCO2H CH2(OH)CO2H reaction 4 CH2(OH)CO2H Draw the structure of X. [1] ZAFA R IQ BAL CH. Pg-23 18. The diagram shows a reaction sequence starting from ethanal. O H 3C C H OH HCN and NaCN H reaction 1 P H2SO4(aq) reaction 2 H3C C H H reaction 3 C CO2H ethanal C CO2H H Q R reaction 4 O H 3C C CO2H S (a) (i) Draw the displayed formula of P. [1] (ii) Name the type of chemical reaction that occurs in reaction 3. ....................................................................................................................................... [1] (iii) Write an equation to represent reaction 4. Use [O] to represent the oxidising agent. ....................................................................................................................................... [1] (iv) State the reagents and conditions for reaction 4. ....................................................................................................................................... [1] ZAFA R IQ BAL CH. Pg-24 19. Twopossiblemethodsofmakinglacticacidareshown. OH O C H3 C reaction 1 H H3C C H CN reaction 2 OH H 3C OH H3C C reaction 3 CH2OH H3 C C H CO2H O H C lactic acid reaction 4 CO2H (ii) Statesuitablereagentsandconditionsforreactions1 and 3. reaction reagents and conditions 1 3 [4] (iii) Namethetypeofreactionthatoccursinreaction2. .......................................................................................................................................[1] (iv) Reaction 4 uses NaBH4. IdentifytheroleofNaBH4 in this reaction. .......................................................................................................................................[1] (v) Lacticacidhasachiralcentre. Statewhatismeantbythetermchiral centre. ............................................................................................................................................. ............................................................................................................................................. .......................................................................................................................................[1] ZAFA R IQ BAL CH Pg-25 ORGANIC SYNTHESIS ALKYL HYDROGENSULPHATE KETONE 2y alcohol ALKENE ALKANE ALCOHOL HALOALKANE AMINE ALDEHYDE NITRILE AMIDE CARBOXYLIC ACID ESTER 1y alcohol 2-HYDROXYNITRILE ZAFA R IQ BAL CH. Pg-26 (e) V is used in a wide range of organic reactions. Some reactions of V are shown. W V O reaction 3 O alkaline aqueous I2 O– reaction 4 NaBH4 Y X OH (i) reaction 5 reaction 6 dehydration addition polymerisation Z V and W are colourless and soluble in water. State what you would observe in reaction 3. ....................................................................................................................................... [1] 9701/22/O/N/21 [Turn over Pg-27 (ii) Reaction 3 is a redox reaction. Identify which of the reactants is reduced in this reaction. ....................................................................................................................................... [1] (iii) Construct an equation for reaction 4. Use [H] in the equation to represent an atom of hydrogen from NaBH4. C6H12O + ..................................................................................................................... [1] (iv) X is a mixture of two optical isomers. Draw the two optical isomers in the boxes provided. (v) Both optical isomers of X can be dehydrated to form a single product, Y. Give the reagent(s) and conditions required for reaction 5. [2] ....................................................................................................................................... [1] (vi) Y can form an addition polymer Z. Draw one repeat unit of Z. (vii) Reaction 6 does not proceed quickly at room temperature. [1] Suggest why this is the case. ............................................................................................................................................. ....................................................................................................................................... [1] [Total: 17] Pg-28 (c) Citric acid can be made from M in a four-step reaction. Cl M Cl O step 1 O Cl N step 2 OH HO HO Cl Cl Cl step 3 citric acid O O HO N OH O O step 4 OH OH OH HO N Complete the table for each step of the reaction sequence to identify: ● the reagents and conditions required ● the type of reaction. step reagent and conditions type of reaction 1 2 dilute sulfuric acid 3 4 dilute sulfuric acid [5] [Total: 12] © UCLES 2021 9701/23/M/J/21 [Turn over Pg-29 4 Some reactions of compound G are shown. G O OH H reaction 1 HOOC(CH2)2COOH reaction 2 O reaction 3 Tollens’ reagent H2SO4, heat under reflux reaction 4 Na H O (a) (i) OH HO O State the type of reaction that occurs in reaction 1. ....................................................................................................................................... [1] (ii) Suggest the reagent(s) and conditions required for reaction 1. ............................................................................................................................................. ....................................................................................................................................... [2] (iii) Draw the structure of the organic product, H, from reaction 2. [1] (iv) State what you would observe in reaction 3. ....................................................................................................................................... [1] (v) Give the type of reaction shown by reaction 4. ....................................................................................................................................... [1] © UCLES 2020 9701/22/O/N/20 [Turn over Pg-30 5 The reaction sequence shows how ethene, C2H4, can be converted into other organic molecules. C 2H 4 reaction 1 OH reaction 5 reaction 2 Cl reaction 6 NH2 O reaction 3 HO N reaction 4 heat with dilute acid W (a) Complete the table to give ● the name of the reaction mechanisms of reactions 1 and 6 ● the reagents and conditions required for reactions 1, 2 and 6. reaction name of reagents and conditions name of mechanism 1 2 6 [6] © UCLES 2020 9701/22/M/J/20 Pg-31 (b) In reaction 3 the organic molecule reacts with HCN and a KCN catalyst. (i) Complete the diagram to show the mechanism of the reaction occurring. Include all relevant dipoles, lone pairs and curly arrows in your answer. H H C H 3C O C– N H3C C CN H – O H H 3C C OH CN C C– N N [3] (ii) Name the functional groups present in the product of reaction 3. ....................................................................................................................................... [2] (c) Draw the structure of the organic molecule W formed in reaction 4. [1] [Total: 12] Pg-32 (b) F is an organic molecule which has the molecular formula C3H6O2. When F is heated with NaOH(aq) followed by H2SO4(aq) the products G and H are made. F NaOH(aq) followed C3H6O2 by H2SO4(aq) G + H Separate samples of G and H are added to Na2CO3(aq) sodium metal alkaline aqueous iodine. ● ● ● The observations are described in the table. reagent(s) G H Na2CO3(aq) colourless bubbles of gas produced no visible reaction Na(s) colourless bubbles of gas produced colourless bubbles of gas produced alkaline aqueous iodine no visible reaction yellow precipitate forms (i) Complete the table to identify the functional groups present in F, G and H. functional group F G H [3] (ii) Name the yellow precipitate formed when alkaline aqueous iodine reacts with H. ....................................................................................................................................... [1] (iii) Draw the structures of G and H. G H [2] [Total: 11] © UCLES 2020 9701/23/M/J/20 [Turn over Pg-33 + By the ______________________________of ester H3O /Heat By the _______________________of alkane nitrile + H3O /Heat By the _____________________of alkanoyl halide By the _____________________of alkanamide H2O + H3O /Heat _________ By the _____________of primary alcohol K2Cr2O7 / H+ By the _____________of aldehyde Metal Carbonate Metal Bicarbonate Metal hydroxide _________ Metal oxide oxidation only methanoic & Ethanedioic acid Metal _________ Carboxylic acid K2Cr2O7 / H+ LiAlH4 in dry ether Combustion alcohol + H3O /Heat PX 3 , PX5 , SOX 2