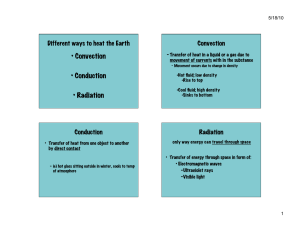
CHAPTER 1 INTRODUCTION MAE 120: Heat and Mass Transfer Bihter Padak Materials used in connection with this course are subject to copyright protection. All course content was created to be used in compliance with the TEACH Act. 17 U.S.C. §110(2) What is Heat Transfer ? Heat : It is the form of energy that can be transferred from one system to another as a result of temperature difference. Heat Transfer : It is the science that deals with the determination of the rates of such energy transfers. Thermodynamics and Heat Transfer They are called thermal sciences. What is the difference? Thermodynamics: It concerns with the amount of heat transfer (between equilibrium states). Ø Time independent Ø Equilibrium phenomenon Ø Deals with the end states of the process Ø Provides no information on the nature of interaction or the time rate at which it occurs Heat Transfer: It deals with the rate of heat transfer. Ø Time dependent processes Ø Non-equilibrium phenomenon Ø Driving force: temperature difference Ø Thermodynamic principles alone are not enough for heat transfer analysis The Difference between Heat Transfer & Thermodynamics How long will it take to cool the coffee in a thermos bottle from 90oC to 70oC? Which one, heat transfer or thermodynamics, can answer this question? Heat transfer can calculate the time needed Some application areas of heat transfer Heat Transfer Mechanisms Heat can be transferred in three different ways: 1. Conduction 2. Convection 3. Radiation Ø All modes of heat transfer require temperature difference (driving force). Ø All modes of heat transfer are from the high temperature medium to the lower temperature one. Conduction Heat Transfer Energy transfers from the more energetic particles to the adjacent less energetic ones as a result of interactions between the particles. Conduction can take place in solids, liquids, or gases In gases and liquids: Conduction is due to collisions & diffusion of molecules In solids: Conduction is due to the combination of vibrations of the molecules in a lattice and the energy transport by free electrons. The rate of heat conduction depends on: Ø geometry Ø material Ø thickness Ø temperature difference Rate of heat conduction through wall: Rate of heat conduction µ dT qcond = −kA dx (J / s or W) k dT/dx A dx (Area) (Temperatu re difference) Thickness Fourier’s Law of Heat Conduction Steady heat transfer through a large plane wall of thickness of Dx and area A = thermal conductivity (W/m.K) = temperature gradient (K/m) = heat transfer area (m2) = thickness of medium in x-direction (m) Heat flux: !! qcond dT = −k dx Heat transfer rate in the xdirection per unit area perpendicular to the direction of heat transfer (W/m 2 ) Convection Heat Transfer The heat transfer between a solid surface and the adjacent fluid (liquid or gas) that is in motion and involves the combined effects of conduction and fluid motion. Convection = Conduction + Advection (fluid motion) There are two convection types: Free Convection: Fluid moving naturally over the surface (density difference) Forced Convection: Fluid is forced to flow over the surface (fan, pump, etc.) Heat transfer from a hot surface to air by convection u The rate of convection heat transfer is expressed by Newton’s law of cooling: qconv = hA (Ts − T∞ ) (J / s or W) A Newton’s Law of Cooling A = heat transfer area between two mediums (m2) h = convection heat transfer coefficient (W/m2.K) Ts = surface temperature of the solid (K) T¥ = temperature of fluid moving over the body (K) qconv = hA (Ts − T∞ ) h is influenced by numerous factors: Ø surface geometry Ø the nature of fluid flow Ø fluid properties Ø the bulk fluid velocity, etc. Radiation Heat Transfer The energy emitted by matter in the form of electromagnetic waves (or photons) as a result of the changes in the electronic configurations of the atoms or molecules. No need of intervening medium. Occurs most efficiently in vacuum. In heat transfer studies we are interested in thermal radiation (radiation emitted by bodies because of their temperature). Ø It is a volumetric phenomenon: all solids, liquids & gases emit, absorb, or transmit radiation Ø For opaque surface solids: it may be considered as a surface phenomenon Radiation Heat Transfer The rate at which energy is released per area (W/m2) è Surface emissive power: E The maximum rate of radiation per area from a surface at an absolute temperature Ts is : 4 s Eb = σ T 2 (W/m ) Stefan-Boltzmann Law TS = surface absolute temperature (K) σ = the Stefan-Boltzmann constant (5.67x10 -8W / m 2 K 4 ) è has to be in K Radiation Heat Transfer Black body: Eb The idealized surface that emits the max. radiation is called “black body” (Black body radiation). For real bodies: The radiation is less and expressed as 4 s E = εσ T 2 (W/m ) e = the emisivity of the surface (0 < e < 1) for black body: e=1 Radiation Heat Transfer Radiation may also be “incident” on a surface from its surroundings Radiation at which all such radiation is incident on a unit area of a surface è Irradiation: G G The absorbed radiation: Gabsorbed = α G Gref = (1− α ) G 0 < a < 1) Gabsorbed = α G a=1: black body (perfect absorber) Absorptivity (a): The fraction of radiation energy incident on a surface that is absorbed by the surface Radiation Heat Transfer The absorbed radiation: Gabsorbed = α G G Gref = (1− α ) G 0 < a < 1) Gabsorbed = α G • a<1 and opaque surface è a portion of radiation is reflected • a<1 and semi transparent surface è a portion of radiation may be transmitted Ø In general: e & a depend on temperature & wavelength of the radiation. Ø Kirchoff’s law of radiation: e & a of a surface at a given temperature & wavelength are equal Ø In many practical applications: average emissivity= average absorptivity xample, the absorptivity of a surface to solar radiation may differ from its to radiation emitted by the walls of a furnace. Special case: Radiation between enclosed surfaces E q"conv e of emissivity orptivity ", and ature Ts (a) 1.6 ndings. E Gas T, h Gas T, h Surroundings at Tsur q"rad q"conv Surface of emissivity ! = " , area A, and temperature Ts Ts > Tsur, Ts > T (b) In case where a surface of emissivity e and Radiation exchange: (a) at a surface (b) between a surface and large surface area A atandabsolute temperature TS is completely enclosed by a much larger (black body) surface at temperature Tsur, the radiation heat transfer between two bodies is: 4 s 4 sur qrad = εσ A(T − T ) Special case: Radiation between enclosed surfaces 4 s 4 sur qrad = εσ A(T − T ) It may be convenient to express as: qrad = hr A(Ts − Tsur ) 2 s 2 sur hr = εσ (Ts + Tsur )(T + T ) hr = the radiation heat transfer coefficient Radiation heat transfer to & from a surface surrounded by a gas can occur in parallel to convection. qtotal = qconv + qrad 4 s 4 sur = hA(T s −T∞ ) + εσ A(T − T ) Simultaneous Heat Transfer Mechanisms In many heat transfer processes heat may be transferred with more than one mechanism. Examples ? Look around and find examples! and energy advection. The first law of thermodynamics addresses total energy, which consists of kinetic and potential energies (together known as mechanical energy) and internal energy. Internal energy can be further subdivided into thermal energy (which will be defined more carefully later) Conservation Laws and Thermal Energy Balance Ø Always define your system Open W Closed Q • tot ∆ Est E in (a) • • E g, E st • E out (b) FIGURE 1.7 Conservation of energy: (a) for a closed system over a time interval and (b) for a control volume at an instant. Ø General balance equation Accumulation = Input – Output + Generation - Consumption • Integral form: Finite time period • Rate form: All terms expressed as rates Conserved properties: total mass, total energy Conservation of mass dM sys = m in − m out dt Conservation of energy dEsys = Ein − E out dt Heat and Other Forms of Energy Ø Energy can exist in numerous forms such as: - Thermal Kinetic Electrical Chemical - Mechanical Potential Magnetic Nuclear Ø Their sum constitutes the total energy of a system. Total Energy of a System (ε) = S all energies Heat and Other Forms of Energy Internal energy: The sum of all microscopic forms of energy is called the internal energy of a system, and is denoted by u (J/kg) or U (J). Microscopic energy: Forms of energy related to the molecular structure of a system and the degree of the molecular activity. Components of Internal Energy Sensible energy/heat: Portion of internal energy associated with translational, rotational and vibrational motion of molecules. Temperature é Sensible heat é Latent energy/heat: The internal energy associated with the phase change Chemical energy: The internal energy associated with the atomic bonds in a molecule Nuclear energy: The internal energy associated with binding forces of nucleus Internal Energy and Enthalpy Ø In the analysis of systems that involve fluid flow, we frequently encounter the combination of properties u and Pn. Ø The combination is defined as enthalpy. h = u + Pv Ø The term Pn represents the flow energy of the fluid (also called the flow work). Specific Heats of Gases, Liquids and Solids Specific heat: Energy required to raise the temperature of a unit mass of substance by one degree (denoted by °C). Ø Two kinds of specific heat (kJ/kg.°C or kJ/kg.K): 1. Specific heat at constant volume (Cv) 2. Specific heat at constant pressure (Cp) Specific Heats of Gases, Liquids and Solids Ø The specific heat of a substance, in general, depend on two independent properties such as temperature and pressure. Ø For an ideal gas, they depend on temperature only. Cp= Cv + R Changes in the internal energy (u) and enthalpy (h) of ideal gases : Differential : du = C V dT and dh = CPdT Finite : Du = C V ,ave DT and Dh = CP,ave DT (J/kg) DU = mC V ,ave DT and DH = mCP,ave DT (J) m = mass of the system (kg) C ave = the average specific heat (kJ/kgo C) Incompressible substance: A substance whose specific volume (or density) does not change with temperature & pressure Ø Volume (density) ¹ f (T, P) Þ CP = C V = C Ø Specific heat depends on temperature only Therefore, for solids and liquids : DU = mCave DT Energy Transfer Energy can be transferred to or from a given mass by two mechanisms: Ø Heat (Q) Ø Work (W) Heat transfer: Driving force is temperature difference (DT ). Q ~ DT Þ Q­, DT­ or Q¯, DT ¯ Heat transfer rate: The amount of heat transferred per unit time (J/s) The First Law of Thermodynamics It says: "Energy can neither be created nor destroyed" Conservation of energy principle: ìTotal energy ü ìTotal energy ü ìChange in the ï ï ï ï ï entering the leaving the í ý í ý = ítotal energy ïsystem ï ïsystem ï ïof the system î þ î þ î εin − εout = Δε sys εin εout Energy can be transferred by heat, work and mass. ε : Total energy ü ï ý ï þ εin = εout The rate form: εin − εout Rate of net energy transfer by heat, work and mass Steady - State: Rate of net energy transfer in by heat, work and mass dε sys = dt (J / s or W) Rate of change in internal, kinetic, potential, etc., energies dε system =0 dt εin = εout Rate of net energy transfer out by heat, work and mass ε = E + E AO Total Thermal+ All other mechanical Thermal and mechanical energy balance: E in − E out + E gen dE sys = dt Thermal and mechanical energy generation Ein − Eout + Egen = ΔEsys change in thermal and mechanical energy of system Integral form Energy Balance for Steady-Flow Systems A large number of devices involve mass flow in and out of the system and are modeled as control volume. Ø Steady - state: No change with time Ø Unsteady-state (Transient): Changes with time Ø Uniform: No change with position In case of steady-flow process: Ein = Eout Þ E tot = constant Under steady conditions, the net rate of energy transfer to a fluid in a control volume is equal to the rate of increasing in the energy of the fluid stream flowing through the control volume. The Surface Energy Balance A special case for which no volume or mass is encompassed by the control surface. E in − E out = 0 • Applies for steady-state and transient conditions. • With no mass and volume, energy storage and generation are not pertinent to the energy balance, even if they occur in the medium bounded by the surface. The Surface Energy Balance Consider surface of wall with heat conduction, convection and radiation. !! − qconv !! − qrad !! = 0 qcond transfer by T1 − T2 4 k − h(T2 − T∞ ) − εσ (T24 − Tsur )=0 L Analysis of Heat Transfer Problems Solution Methodology 1. Known: State briefly what is known. 2. Find: State briefly what must be found. 3. Schematic: Draw a schematic of the physical system. Mark the control volume. Identify relevant HT processes by labeled arrows. 4. Assumptions: List all simplifying assumptions. 5. Properties: Compile property values needed. 6. Analysis: Apply appropriate conservation laws, introduce the rate equations as needed. Develop the analysis as completely as possible before substituting numerical values. Perform the calculations. 7. Comments: Discuss your results (key conclusions, a critique of assumptions, trends, parameter sensitivity). Idealization of a System: Assumptions Modeling of Heat Transfer Problems L (?) Example Problem Statement: The coating on a plate is cured by exposure to an infrared lamp providing an irradiation of 2000W/m2. It absorbs 80% of the irradiation and has an emissivity of 0.50. It is also exposed to an air flow and large surroundings for which temperatures are 20°C and 30°C, respectively. If the convection coefficient between the plate and the ambient air is 15 W/m2.K, what is the cure temperature of the plate? frared lamp. Heat transfer from the coating is by convection to ambient xchange with the surroundings. Solution ind: Coating radiation Known: 1. Cure temperature for h # 15with W/m2 ! K.prescribed properties is cured by irradiation from an 2 2. Effect of airflow on the cure temperature for 2the " h coating " 200 W/m infrared lamp. HT from is ! K which the cureconvection temperature isto 50!C. ambient air and radiation chematic: Find: exchange with the surroundings. Cure temperature for h = 15 W/m2.K. Schematic: Tsur = 30°C Glamp = 2000 W/m2 q"conv T∞ = 20°C 2 ≤ h ≤ 200 W/m2•K Air T Coating, α = 0.8, ε = 0.5 q"rad α Glamp Assumptions: Ø Steady-state conditions Ø Negligible heat loss from back surface Ø No internal heat generation Ø Plate is small object in large surroundings and coating has an absorptivity of a = 0.80. Properties: Analysis: h = 15 W/m2.K, a = 0.80, e = 0.5, Tsur = 30°C, T¥ = 20°C Since the process is at steady-state conditions and there is no HT at the back surface, the plate must be isothermal (Ts=T). A control surface placed about the entire plate and applying energy conservation law will yied the desired temperature. Conservation of Energy: Ein − Eout + Egen = ΔEsystem Steady-state and no internal heat generation: Ein − Eout = 0 "" − qrad "" = 0 (αG)lamp − qconv 4 (αG)lamp − h(T − T∞ ) − εσ (T 4 − Tsur )=0 Insert numerical values and solve for T: 0.8 x 2000 W / m2 - 15 W / m 2K(T - 293 )K - 0.5 x5.67 x10 -8 W/ m 2K 4 (T 4 - 303 4 )K 4 = 0 T = 377 K = 104°C st lamp irradiation by the coating and outflow due to convection and net radiation transfer to the surroundings, it follows that Comments: (!G)lamp # q%conv # q%rad ! 0 Final characteristics of and the including wear and Substituting from Equations 1.3a 1.7, coating, we obtain durability, are known to depend on the4 ) temperature at (!G)lamp # h(T # T!) # "#(T 4 # Tsur !0 which curing occurs. An air flow system is able to control Substituting numerical values the air velocity, hence the convection coefficient, on the 0.8 $ 2000 W/m2 # 15 W/m2 ! K (T # 293) K cured surface. However, the process engineer needs to #8 2 4 4 4 4 # 0.5 $ 5.67 $ 10 W/m ! K (T # 303 ) K ! 0 know how the temperature depends on the convection and solving by trial-and-error, we obtain coefficient. T ! 377 K ! 104"C " Solving the energy conservation equation for selected 2. Solving the foregoing energy balance for selected values of h in the prescribed range values of hthewill result: and plotting results, we obtain 240 200 T (!C) 160 120 80 50 40 0 0 20 40 51 60 h (W/m2•K) 80 100 Let’s see what we have learned! 1. How does heat transfer differ from thermodynamics? 2. What is heat transfer? 3. Can you give a few examples of heat transfer appliances in household or in your classroom? 4. What kinds of energy forms do you remember? 5. What does the first law of thermodynamics say? 6. What is specific heat? What is its unit? 7. How many specific heats are defined in thermodynamics ? 8. What are the mechanisms of heat transfer? How are they distinguished from each other? 9. What is a blackbody? How do real bodies differ from blackbody?