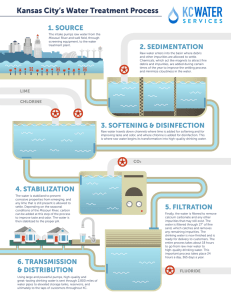
Module 4: WATER PROCESSING Reference: Hammer, M.J. & Hammer, M.J.J. (2014). Water and Wastewater Technology,7th edition. London: Pearson Education Limited. I. Chlorination • the process of adding the element chlorine to water as a method of water purification to make it fit for human consumption as drinking water (disinfection) • oxidizes dissolved iron and manganese in water (color removal) Fe+2 + Mn+2 + O2 FeOx + MnO2 I. Chlorination Chlorine - symbol Cl, greenishyellow gaseous element - strong oxidizing agent, reacting with water, organic compounds, and many metals I. Chlorination Commonly used forms: 1. Calcium Hypochlorites (Ca(OCl)2) • • • Dry form Granular, powdered or tablet foems Readily dissolves in water and contain 70% available chlorine 2. Sodium Hypochlorite (NaOCl) • • • Liquid form 5-15% available chlorine Cheaper Available chlorine – chlorine existing in water as hypochlorous acid and hypochlorite I. Chlorination pH>8 Cl2 + H2O HCl + HOCl H+ + OClpH<7 Ca(OCl)2 + H2O Ca+2 + 2OCl + H2O if the pH is between 4.5 – 8.5: HOCl + NH3 H2O + NH2Cl (monochloroamine) HOCl+NH2Cl H2O + NHCl2 (dichloroamine) if the pH is below 4.4: HOCl+NH2Cl2 H2O + NCl3 (trichloroamine) I. Chlorination Free available residual chlorine is that residual chlorine existing in water as hypochlorous acid or hypochlorite ion. Combined available residual chlorine is that residual existing in chemical combination with ammonia (chloramines) or organic nitrogen compounds. Chlorine demand is the difference between the amount added to a water and the quantity of free and combined available chlorine remaining at the end of a specified contact period. I. Chlorination Example 1. I. Chlorination Example 2. II. Aeration • the saturation of water with air, water is brought into contact with air in such a manner as to produce maximum diffusion, usually by spraying water into the air in fountains to remove dissolved gases • removes odors and taste caused by decomposing organic matter, and also industrial wastes such as phenols and volatile gases such as chlorine • it also converts dissolved iron and manganese compounds into insoluble hydrated oxides of the metals which may then be readily settled out III. Softening • Removal of hardness • Maximum level for public supply – 300 to 500 mg/L • Hardness interferes with laundering and may produce scaling in pipes • Precipitation softening – uses lime (Ca)) and soda ash (Na2CO3) • Other benefits of Lime treatment: • Bactericidal action • Removal of iron • Aids in clarifying turbid waters III. Softening • Forms of lime: • Quicklime • granular form • Minimum of 90% CaO (MgO is primary impurity) • Hydrated lime • Powdered form • Approximately 68% CaO • Lime slurry (Ca(OH)2) • Soda ash – grayish white powder - contains at least 98% sodium carbonate III. Softening Note: precipitation of Mg ion demands a high pH and the presence of excess lime (+35 mg/L CaO) III. Softening Example 3. Water defined by the following analysis is to be softened by excess lime treatment. CO2 = 8.8 mg/L Alk (HCO3-) = 135 mg/L as CaCO3 Ca+2 = 40 mg/L SO4 -2 = 29 mg/L Mg+2 = 14.7 mg/L Cl= 17.8 mg/L Na+ = 13.7 mg/L A) Sketch a meq/L bar graph and list the hypothetical combinations of chemical compounds in solution. B) Calculate the softening chemicals required, expressing lime dosage as CaO and soda ash as Na2CO3. III. Softening Example 3 Solution. a. the figure III. Softening Example 3 Solution. b. IV. Mixing and Flocculation Recommended detention time for rapid mixing: <30 sec IV. Mixing and Flocculation • causes the suspended solids to coalesce IV Mixing and Flocculation Recommendations for flocculation basins: • Inlet and outlet shall prevent short-circuiting and destruction of floc • Min flow-through velocity: 0.5-1.5 ft/min (2.5-7.5 mm/s) • Detetion time: > 30 min • Agitator speed: 0.5-3.0 ft/s (0.15-0.91 m/s) • Must be close together as possible with sedimentation basins • Velocity through conduits: 0.5-1.5 ft/s (0.15-0.45 m/s) • Allowances made to reduce turbulence at bends IV. Mixing and Flocculation Example 4. Laboratory tests were conducted on the chemical treatment of a water using both a long, narrow flocculation tank and a complete mixing unit. The observed rate constants under steady state conditions were first order kinetics equal to “6 per day” for plug flow and “18 per day” for complete mixing. If the desired degree of removal is 90% for an influent concentration of 60 mg/L, which process requires the shortest retention time? Solution: C0 = 60 mg/L For 90% removal, Ct =(1-0.9)60 mg/L = 6 mg/L IV. Mixing and Flocculation V. Coagulation the process of adding chemicals to the wastewater which causes the surface characteristics of the suspended solids to be altered so that they attach to one another and precipitate • Commonly used coagulants: • Aluminum based (aluminum sulfate, sodium aluminate, potash alum, ammonia alum) • Iron based (ferric sulfate, ferrous sulfate, chlorinated ferrous sulfate, ferric chloride) V. Coagulation • Aluminum Sulfate • Commonly known as alum, filter alum or alumina sulfate • Al2(SO4)3 XH2O • Grayish white crystallized solid • lump, ground or powdered form or • concentrated solution • Most widely used V. Coagulation Aluminum Sulfate reactions with (5) natural alkalinity, (6) lime and (7) soda ash 1.0 mg/L of alum(MW=600) reacts with 0.5 mg/L natural alkalinity (as CaCO3) 0.39 mg/L of 95% hydrated lime as Ca(OH)2 0.33 mg/L 85% quicklime 0.53 mg/L soda ash as Na2CO3 V. Coagulation Example 5. V. Coagulation Example 6 VI. Sedimentation or Clarification • removal of particulate matter, chemical floc and precipitates from suspension through gravity settling VI. Sedimentation or Clarification t=V/Q vo = Q / A weir loading = Q / L where : t = detention time V = basin volume Q = volumetric flow rate vo = overflow rate A = cross-sectional area L = weir length VI. Sedimentation or Clarification vsettling > Q/A heavier flocs removed vsettling < Q/A lighter flocs carried out in the basin effluent VI. Sedimentation or Clarification Recommendations for sedimentation basins: • Maximum horizontal velocity: 0.5 ft/min (2.5 mm/s) • Detention time: > 4 hours • Maximum weir loading: 20000 gpd/ft of weir length (250 m3/m-day) • Overflow rate: 500-800 gpd/sq ft (20-33 m3/m-day) VI. Sedimentation or Clarification Example 7. VI. Sedimentation or Clarification Example 7 Solution. VI. Sedimentation or Clarification Example 7 Solution. VII. Filtration • process of separating a suspended solid, such as a precipitate, from the liquid in which it is already suspended by straining it through a porous medium that can be penetrated easily by liquids • used to remove nonsettleable floc remaining after chemical coagulation and sedimentation VII. Filtration 1. 2. 3. 4. Filtration: Valve 1,4 open ; Valves 2, 3, 5 close Backwashing1: Valve 2 open; Valves 1, 3, 4, 5 close Backwashing2: Valve 2,5 open; Valves 1, 3, 4 close Before next run: Valves 1,3 open; Valves 2, 4, 5 close VII. Filtration Kinds of Filter Media: 1. SAND - loose, incoherent mass of mineral materials in a finely granular condition usually consisting of quartz (silica), with a small proportion of mica, feldspar, magnetite, and other resistant minerals 2. COAL - a combustible organic rock composed primarily of carbon, hydrogen, and oxygen; porous surface makes it a good adsorbent 3. GARNET - group of related minerals, often used as gemstones or abrasives; garnets are compound silicates VII. Filtration Example 8. A filter unit is 15 ft by 30 ft. After filtering 2.5 mil gal in a 24-hr period, the filter is backwashed at a rate of 15 gpm/sq ft for 12 min. Compute the average filtration rate and the quantity and percentage of treated water used in backwashing. VIII. Ion-exchange • method of exchanging ions in a solution with ions of the same charge in certain insoluble substances • by this means chemicals can be removed from a solution that contains large amounts of other chemicals • this is done by passing the solution through porous solid materials, usually minerals of the zeolite group or specially prepared synthetic resins (plastics) containing large, complex molecules VIII. Ion-exchange Kinds of Cation exchanger: 1. Natural greensand (glauconite) - dull green mineral used in water softening - it forms in sands and sandstones as grains or pellets - chemically, it is a hydrous iron-potassium silicate - has a value of 2 on the Mohs hardness scale and a specific gravity of about 2.3 VIII. Ion-exchange Kinds of Cation exchanger: 2. Silicon gel-type zeolite - large group of minerals composed of hydrated aluminum - - silicates of alkali metals and alkaline earth metals named because of their swelling and bubbling under high temperature range in hardness from 3 to 6 and have a specific gravity of 1.9 to 2.8 are usually found in veins and cavities of basic igneous rocks, especially basalt are used as water-softening agents IX. Carbon Adsorption Taste and color removal using activated carbon IX. Carbon Adsorption Activated Carbon - prepared from hardwood charcoal, lignite, nut shells, or other carbonaceous materials by controlled combustion - Each particle is honeycombed with thousands of molecular-sized pores - Available in powdered or granular form X. Fluoridation • the practice of adding fluoride compounds to water with the intended purpose of reducing tooth decay in the general population • Optimum concentration between 0.6 and 1.2 ppm • Commonly used in water treatment: sodium fluoride, sodium silicofluoride and fluorosilicic acids (also known as hydrofluorosilicic acid, hexafluorosilicic or silicofluoric acid) X. Fluoridation Image references: 1. https://www.alibaba.com/productdetail/High-Purity-Optical-SodiumFluoride-for_50039690751.html 2. http://pureborax.sell.everychina.com/p107925065-99-disodiumhexafluorosilicate-odorless-sodiumsilicofluoride-suppliers.html 3. https://www.a2zpressrelease.com/che micals-and-materials/fluorosilicic-acidmarket-research-report-2019-2023/ X. Fluoridation Example 3 X. Fluoridation Example 4 THANK YOU FOR LISTENING!