Heterocyclic Compounds: Pyridine, Pyrrole, Indole, Quinoline
advertisement
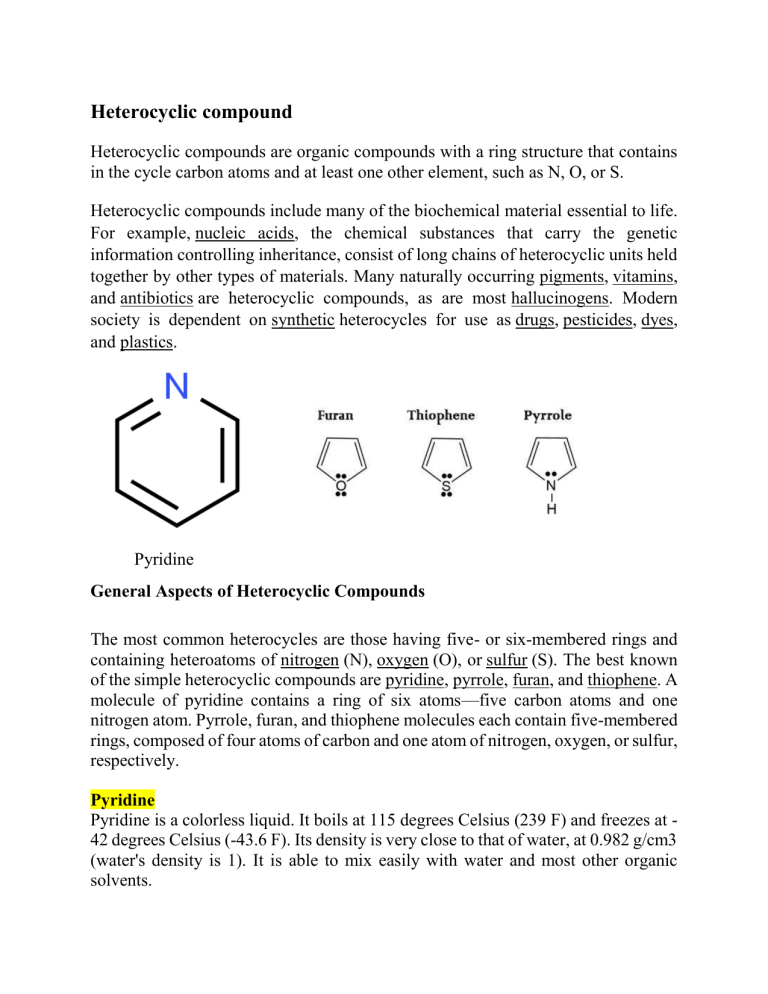
Heterocyclic compound Heterocyclic compounds are organic compounds with a ring structure that contains in the cycle carbon atoms and at least one other element, such as N, O, or S. Heterocyclic compounds include many of the biochemical material essential to life. For example, nucleic acids, the chemical substances that carry the genetic information controlling inheritance, consist of long chains of heterocyclic units held together by other types of materials. Many naturally occurring pigments, vitamins, and antibiotics are heterocyclic compounds, as are most hallucinogens. Modern society is dependent on synthetic heterocycles for use as drugs, pesticides, dyes, and plastics. Pyridine General Aspects of Heterocyclic Compounds The most common heterocycles are those having five- or six-membered rings and containing heteroatoms of nitrogen (N), oxygen (O), or sulfur (S). The best known of the simple heterocyclic compounds are pyridine, pyrrole, furan, and thiophene. A molecule of pyridine contains a ring of six atoms—five carbon atoms and one nitrogen atom. Pyrrole, furan, and thiophene molecules each contain five-membered rings, composed of four atoms of carbon and one atom of nitrogen, oxygen, or sulfur, respectively. Pyridine Pyridine is a colorless liquid. It boils at 115 degrees Celsius (239 F) and freezes at 42 degrees Celsius (-43.6 F). Its density is very close to that of water, at 0.982 g/cm3 (water's density is 1). It is able to mix easily with water and most other organic solvents. Pyridine and pyrrole are both nitrogen heterocycles—their molecules contain nitrogen atoms along with carbon atoms in the rings. The molecules of many biological materials consist in part of pyridine and pyrrole rings, and such materials yield small amounts of pyridine and pyrrole upon strong heating. In fact, both of these substances were discovered in the 1850s in an oily mixture formed by strong heating of bones. Today, pyridine and pyrrole are prepared by synthetic reactions. Their chief commercial interest lies in their conversion to other substances, chiefly dyestuffs and drugs. Pyridine is used also as a solvent, a waterproofing agent, a rubber additive, an alcohol denaturant, and a dyeing adjunct. Pyrrole characterized by a ring structure composed of four carbon atoms and one nitrogen atom. The simplest member of the pyrrole family is pyrrole itself, a compound with molecular formula C4H5N. The pyrrole ring system is present in the amino acids proline and hydroxyproline; and in coloured natural products, such as chlorophyll, heme (a part of hemoglobin), and the bile pigments. Pyrrole compounds also are found among the alkaloids, a large class of alkaline organic nitrogen compounds produced by plants. Uses of pyrrole Pyrrole is a precursor to the drug tolmetin. Polypyrrole is of some commercial value. N-Methylpyrrole is a precursor to N-methylpyrrolecarboxylic acid, a building-block in pharmaceutical chemistry. Pyrroles are also found in several drugs, including atorvastatin, ketorolac etc. Pyrroles are used as lightfast red, scarlet, and carmine pigments.[ Indole a colorless to yellowish solid, C8H7N, having a low melting point and a fecal odor, found in the oil of jasmine and clove and as a putrefaction product from animals' intestines: used in perfumery and as a reagent. Uses Indole-3-carbinol is a substance found in vegetables such as broccoli, Brussels sprouts, cabbage, collards, cauliflower, kale, mustard greens, turnips, and rutabagas. It can also be produced in the laboratory. Indole-3-carbinol is used for prevention of breast cancer, colon cancer, and other types of cancer. The National Institutes of Health (NIH) has reviewed indole-3carbinol as a possible cancer preventive agent and is now sponsoring clinical research for breast cancer prevention. Structure of Indole Quinoline Quinoline, any of a class of organic compounds of the aromatic heterocyclic series characterized by a double-ring structure composed of a benzene and a pyridine ring fused at two adjacent carbon atoms. The benzene ring contains six carbon atoms, while the pyridine ring contains five carbon atoms and a nitrogen atom. The simplest member of the quinoline family is quinoline itself, a compound with molecular structure C9H7N. Uses of Quinoline Quinoline is used principally for the manufacture of nicotinic acid, which prevents pellagra in humans, and other chemicals. Several methods are known for its preparation, and production of synthetic quinoline exceeds that from coal tar. Several alkaloids (alkaline organic compounds produced in plants) are members of the quinoline family; these include quinine and other derivatives from the cinchona tree. The antimalarial drugs chloroquine, mefloquine, and amodiaquin are synthetic quinoline compounds, as are dibucaine hydrochloride, a long-acting local anesthetic that is commonly used as a topical agent to relieve pain from minor cuts and insect bites and stings, and cyanine, the oldest of an important class of dyes. Structure of Quinoline





