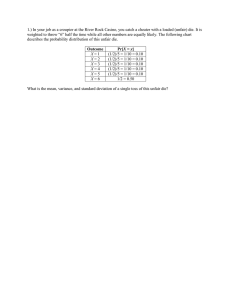
Chapter 4 - POIYDER METALLURGY
Introduction
Powder Melallarg is the name given to the process by which fine powdered
materials are blended, pressed into a desired shape (compacted), and then heated
(sintered) in a controlled atmosphere to bond the contacting surfaces ofthe
particles and establish the desired properties, The process commonly designated
as P/M, readily lends itself to the mass production of small, intricate parts of high
precision, often eliminating the need for additional machining or finishing. The
powder metallurgy process is both simple and complex: simple because the
operations required to produce a component me relatively easy to understand; complex because the more precise the
characteristics of the component (dimensions, mechanical attributes, corosion resistance, etc.), the more diffrcult
the work becomes. Contrary to raw material machining, powder metallurgy uses powdered materials, which are as
fine as flour or icing sugar. The contol of mechanical and physical properties increases in complexity depending on
the required characteristics.
Historical Background
While a crude form of powder metallurgy appears to have existed in Egypt as early as 3000 B.C., mass
manufacturing of P/M products did not begin until the mid or late nineteenth century. As of this time, powder
metallurgy was used to produce copper coins and medallions, platinum ingots, and tungsten wires. the primary
material for light bulb filaments early in the twentieth century. By the 1920s tungsten carbide cutting-tool tips and
nonferrous bushings were being produced. Self-lubricating bearings and metallic filters were other early products. A
period of rapid technological development occurred after World War II based primarily on automotive applications,
and iron and steel replaced copper as the dominant P/l\4 material Aerospace and nuclear developments created
accelerated development for refractory and reactive materials where P/M processing is quite atffactive. Full-density
products emerged in the 1960s, and high-performance superalloy components, such as aircraft turbine engine parts,
were a highlight of the 1970s. Developments in the 1980s included the commercialization of rapidly-solidifie{ and
amorphous powders and the development of P/IvI injection molding technology.
Basic Processes
Powders are blended with lubricants for particle to particle and die wall lubrication and then tested for their
characteristics before being placed in production. This is necessary to produce the part to exacting size and strength
specifications.
The powder metallurgy process generally consists of four basic steps:
l. Powder Manufacture
2.
3.
4.
Additives
(Lubricants or Binders)
Mixing or Blending
Compacting
Sintering
Optional Secondary
Manufaciuring
Simplified Flowchart of the Powder Metallurgy Process
The properties of powder metallurgy products are highly dependent on the characteristics of the metal powders that
are used. Some important properties and characteristics include chemistry and purity, particle size, size distribution,
particle shape, and the surface texture ofthe particles.
Several processes can be used to produce powdered material, with each imparting distinct properties and
characteristics to the powder and hence to the final product. The following are the methods used for producing metal
powders:
L
Air, nitrogen and argon are commonly
used
gases, and water is the liquid most widely used.
Shapes of gas atomization powders
{clkr:l:S
i
Vertirol
Efl
s
+'liistr
itlotri:altn
1..:lrr.rrrr.. .'/.
By varying the several parameters: design and configurations of the jets. pressure and volume of the
atomizing fluid, thickness of the stream of metal etc. - it is possible to control the particle size distribution
over a wide range. The particle shape is determined largely by the rate of solidification and varies from
spherical, if a low heat capacity gas is employed, to highly irregular if water is used. In principle the
technique is applicable to all metals that can be melted, and is commercially used for the production of
iron, copper, including tool steels, alloy steels, brass, bronze and the low-melting-point metals, such as
aluminum, tin, lead zinc, cadmium. The readily oxidizable metals, for example chromium-bearing alloys,
are being atomized on an increasing scale by means of inert gas, specially argon.
WATER ATOMIZATION
Shapes of water atomization powders
Water atomization
2
2.
Alomizatlonfrom o rotating consamable eleclrode
In this method, an eleitric arc impinges on a rapidly rotating electrode (all contained within a chamber
purged with inert gas), with centrifugal force causing the molten droplets to fly from the surface of the
electrode.
There are basieally two types of centrifugal atomization processes:
,
in one a cup of molten metal is rotated on a vertical axis at a speed sufficient to throw offdroplets of
molten metal, or a stream of metal is allowed to fall on a rotating disc or cone;
in the other a bar of the metal is rotated at high speed and the free end is progressively melted e.g. by
an electron beam or plasma arc.
This latter procers is called the Rotating Electrode Process (REP), and the bar may be rotated either on a
horizontal or on a vertical axis. A special advantage ofthese processes is that they can be carried out in a
sealed vessel in a controlled atmosphere - even vacuum - and thus produce 'clean' powders of highly
reactive metals. With the REP process the avoidance of contact with refractory is a potent means of
reducing the number of non-metallic inclusions in the powder, and in components manufactured from the
powder.
Atomization is particularly useful for the production of alloys in powder form, since the constituenr, -.t"1,
are fully alloyed in the molten state, Thus each powder particle has the same chemical composition
Additionally the process is used to produce compositions such as copper-lead, in which the lead, though
soluble in the liquid state, comes out of solution on solidification. If a casting of such an alloy is made,
serious segregation of the lead results, but if the liquid is atomized, the end product is copper powder
containing a very fine and uniform distribution of lead inclusions within each powder particle.
,
Other Methods includes
a. Chemical Reduction of Paniculate Compourds (generally crushed oxides or ores)
b. Elecfiolytic Depositionfrom Solutlons or Fused Salts
e.
d.
e.
Pulverizotlon or Grindlng of Brltlle Materlals
Thermal Decomposilion of Hydrides or Carbonyls, Precipitatlonfrom Solution,and
Condensatlon of Meld Yapon
Powder llllxing And Blending
It is the process of mixing together the metal powders and lubricants (or binders) with alloy addition until they are
thoroughly blended. Some powders, such as graphite, can even play a dual role, serving as a lubricant during
compacting and a source of carbon as it alloys with iron during sintering to produce steel. Lubricants such as
graphite improve the flow characteristics and compressibility at the expense of reduced green strength. Binders
produce the reverse effect.
Green Strength is used'to describe the strength of the pressed powder immediately after compacting.
Compacting
One of the most critical steps in PlN4 process is compacting. In compacting, the
blended metal powder is fed into a precision die, compressed and densified
into a shape known as green compact, usually at room temperature with
pressnre ranging from 3 to 120 tonVin2 depending on material and application.
Metal'forming processes, such as rolling, forging, extrusion, and swagging,
have also been adapted to compact powders.
The shape then has sufficient coherence and strength to be ejected from the die
and can be handled like any machined part. The degree to which the powder is
compressed controls the density or weight per unit volume of the pressed part.
P..."'
It confrols to a great extent the amount of shrinkage or growth during sintering
and the physical properties ofthe parts that are developed. Therefore, close control ofthe density ofthe pressed
powder part is very important. All compacts produced ftom a particular die, from the same powder and under the
same compacting conditions will be identical in dimensions. Very complex shapes can be formed repeatedly and
,
accurately.
Sintering
ln the sintering operation, the pressed-powder compacts
are heated in a
controlled-atmosphere environment to a temperature below the melting
point but high enough to permit solid-state diffusion, and held for
sufficient time to permit bonding of particles. Most metals are sintered
at temperatures of 70 to 80%o of their melting point, while refractory
materials may require temperature neat 90o/o. When the product is
composed of more than one material, the sintering temperatur€ may
even be above the melting temperature of one or more components.
The lower-melting-point materials then melt and flow into the voids
between the remaining palticles.
Most sintering operations involve three stages. These are:
1. Burn-offor Purge Stage
Is designed to combust any air, volatilize and remove lubricants or binders that would interfere
with good bonding, and slowly raise the temperafure of the compacts in a controlled manner
.,
3.
High-temperature Stage
It is the second stage where the desired solid-state diffusion and bonding between the powder
particles take place. The time must be sufficient to produce the desired densiry and final
properties, and usually varies from l0 minutes to several hours.
Cooling Period
This stage is required to lower the temperature of the products while maintaining them in a
controlled atmosphere. This feature serves to prevent both oxidation that would occur upon direct
discharge into air and possible thermal shock fiom rapid cooling.
Secondary operations
Powder Blending and Lubrication
C o i n i ng-S iz
ing- Repr e s s ing
o
$q
Secondary Machining
Powder Production
4
The different steps of the P/}vI manufacturing process can be described as follow
:
* ;/' rP
<d;l
Base pmdff.
brod:ng and lubn;ants
Pra6ring
?
iG,
w
Final part
Advantages Of Powder Metallurgy
I.Eliminatlon or reducli.on of machining
The dimensional accuracy and surface finish of P/]vI products are such that subsequent machining operations can
be totally eliminated for many applications. If unusual dimensional accuracy is required, simple coining or sizing
operations can often give accuracies equivalent to those of most production machining.
2.
High productlon rutes
All
steps in the P/lv1 process are simple and readily automated. Labor requirements are low, and product
uniformity and reproducibility are among the highest in manufacturing.
3.Complex shopes can be produced
Subject to the limitations discussed previously, complex shapes can be produced, such as combination gears,
cams, and internal keys. It is often possible to produce parts by powder metallurgy that cannot be machined or
cast economically.
l.llide
varlotlons ln composlttons are posslble
Parts of very high purity can readily be produced. Metals and ceramics can be intimately mixed. Immiscible
materials can be combined, and solubility limits can be exceeded. In most cases the chemical homogeneity of the
product exceeds that ofall competing techniques.
5. Wide voriations in properties are available
Products can range from low-density parts with controlled permeability to high-density parts with properties that
equal or exceed those of equivalent wrought counterparts. Damping of noise and vibration can be tailored into a
P/M product. Magrretic properties, wear properties, and others can all be designed to match the needs of a specific
application.
6.Scrap ls ellminated or rcduced
Powder metallurgy is the only common manufacturing process-in which no material is wasted. In casting,
machining, and press forming, the scrap can often exceed 5oo/o of the starting material. This is particularly
important where expensive materials are involved and may make it possible to use more costly materials without
increasing the overall cost of the product. An example of such a product would be the rare-earth magnets.
5
Disadvantages Of Powder Metallurgy
l. Inferior strength properties
Because of the residual porosity, powder metallurgy parts generally have mechanical properties that are inferior to
wrought or cast products of the same material. Their use may be limited when high stresses are involved.
However, if the additional expense is justified, the required strength and fracture resistance can often be obtained
by using different materials or by employing altemate or secondary techniques.
2, Relative high die cost
Because
of the high pressures and severe abrasion involved in the process, the P/IvI dies must be made of
of
expensive material and be relatively massive. Because of the need for part-specific tooling, production volumes
less than 10,000 identical parts are normally not practical.
3, High materiol cost
On a unit weight basis, powered metals are considerably more expensive than wrought or cast stock. However. the
of scrap and the elimination of machining can often offset the higher cost of the starting material. In
addition, P/M is usually employed for rather small parts where the material cost per part is not very great.
absence
4.
Design limitations
The powder metallurgy process is simply not feasible for many shapes. Parts must be able to be ejected from the
die. The thickness/diameter (or thickness/width) ration is limited. Thin vertical sections are difficult, and the
overall size must be within the capaciry of available presses.
5.
Density voriations ptoduce property variotions
The non-uniform product density that is frequently produced in compacting operations generally results in
property variations throughout the part. For some products, these variations would be unacceptable.
6.
Health ond safety hozprd
Many metal, such as aluminum, titanium, magnesium, and iron, are pyrophoric-they can ignite or explode when in
particle form with large surface/volume ratios. Fine particles can also remain airborne for long times and can be
inhaled by rvorkers. To minimize the health and safety hazards, the handling of metal powders frequently requires
the use of inert atmospheres, dry boxes, and hoods, as well as special cleanliness of the working environment.
6