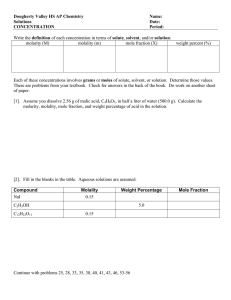
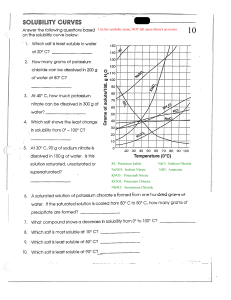
1. What is the molality of a solution that contains 13.4 grams of calcium chloride dissolved in 655 mL of water? Density of H2O 1 g/mL 2. How many mL of hydrogen peroxide are needed to make a 12.5% solution by volume of hydrogen peroxide if you want to make 320 mL of solution? 40 mL 3. What is the mole fraction of the solute in the solution from problem 2? *note that the density of H2O2 is 1.45 g/mL .09 4. What is the mole fraction of the solvent in the solution from problem 2? *note that the density of H2O is 1 g/mL .91 5. You want to create a 0.25 m Potassium Chloride (KCl) solution. You mass 5.00 grams of Potassium Chloride. How much water is needed? .272 6. What is the molality of a solution that contains 48 grams of sodium chloride (NaCl) and 250 mL of water? *note: the density of water is 1 g/mL 7. What is the percentage by mass of the solution from problem 6? 19.2