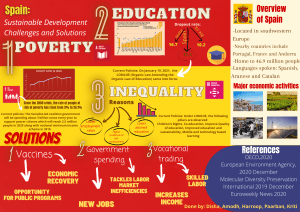
Coordination compounds #BOUNCEBACK Sakshi Vora IIT Roorkee ❏ 7+ years Teaching experience ❏ 10th, 12th CBSE State Topper ❏ KVPY fellow Join with us in Telegram Telegram Channel ● t.me/unacademyatoms Complete Notes and Lectures ● livedaily.me/atoms Study Planner Personal Guidance Customized study plan with bi-weekly reviews Get one on one guidance from top exam experts Live Classes ICONIC Weekly Tests PLUS Structured Courses Unlimited Access Test Analysis Get one on one guidance from top exam experts Study Material Specialised Notes & Practice Sets Experts' Guidelines Study booster workshops by exam experts SAKSHI SAKSHI Coordination Compounds A. B. C. D. [Main Sep. 05, 2020(II)] A. B. C. D. [Main Sep. 03, 2020 (I)] A. B. C. D. [Main Sep. 03, 2020 (II)] A. B. C. D. [Main Sep. 02, 2020 (II)] A. B. C. D. [Main Jan. 09, 2020 (I)] A. B. C. D. [Main Jan. 09, 2020 (II)] A. B. C. D. [Main Jan. 08, 2020 (I)] [Main Jan. 08, 2020 (II)] A. B. C. D. A. B. C. D. [Main Jan. 07, 2020 (I)] A. B. C. D. [Main Jan. 07, 2020 (II)] A. B. C. D. [Main Jan. 07, 2020 (II)] A. B. C. D. [Main April 12, 2019 (II)] A. B. C. D. [Main April 9, 2019 (II)] A. 14 B. 6 C. 8 D. 10 [Main Jan. 11, 2019 (II)] A. B. C. D. [Main Jan. 10, 2019 (II)] A. B. C. D. [Main 2018] A. 16 B. 12 C. 8 D. 24 [Main April 15, 2018 (II)] [Main Sep 05, 2020 (I)] [Adv. 2019] [Adv. 2016] [Adv. 2015] [Adv. 2015] [Adv. 2015] A. B. C. D. [Adv. 2013] A. B. C. D. [Main Sep 06, 2020 (I)] A. B. C. D. [Main Sep 06, 2020 (II)] A. B. C. D. [Main Sep 05, 2020 (I)] A. B. C. D. [Main Sep 04, 2020 (I)] A. B. C. D. [Main Sep 04, 2020 (II)] A. B. C. D. [Main Sep 04, 2020 (II)] A. 145.5 B. 242.5 C. 83.7 D. 97 [Main Sep 03, 2020 (I)] The d-electron configuration of [Ru(en)3]Cl2 and [Fe(H2O)6]Cl2, respectively are : A. B. C. D. [Main Sep 03, 2020 (II)] A. B. C. D. [Main Sep 02, 2020 (I)] [Main Sep 02, 2020 (I)] A. B. C. D. A. B. C. D. [Main Jan 09, 2020 (I)] A. B. C. D. [Main Jan 09, 2020 (II)] A. B. C. D. [Main Jan 08, 2020 (II)] A. B. C. D. [Main Jan 07, 2020 (I)] A. B. C. D. [Main April 12, 2019 (I)] A. B. C. D. [Main April 9, 2019 (I)] A. B. C. D. [Main April 8, 2019 (I)] A. Mn-C bond B. Mn-Mn bond C. Mn-O bond D. C-O bond [Main Jan 12, 2019 (I)] A. Enthylenediamine B. CN- C. NCS- D. CO [Main Jan 12, 2019 (II)] The number of bridging CO ligand(s) and Co-Co bond(s) in CO2(CO)8, respectively are : A. 2 and 1 B. 2 and 0 C. 0 and 2 D. 4 and 0 [Main Jan. 11, 2019 (II)] Wilkinson catalyst is : A. [(Ph3P)3IrCl] B. [(Et3P)3RhCl] C. [(Ph3P)3RhCl] D. [(Et3P)3IrCl] [Main Jan. 10, 2019 (I)] A. B. C. D. [Main Jan. 9, 2019 (II)] A. B. C. D. [Main April 16, 2018] A. B. C. D. [JEE Adv.2016] A. R<Q<P B. Q<R<P C. R<P<Q D. Q<P<R [Adv. 2013] [Adv. 2018] [Main Sep. 05, 2020 (II)] [Main Jan. 08, 2020 (I)] [Main Jan. 08, 2020 (II)] A. B. C. D. [Adv. 2020] [Adv. 2018] A. It has two geometrical isomers B. It will have three geometrical isomers if bidentate ‘en’ is replaced by two cyanide ligands. C. It is paramagnetic D. It absorbs light at longerwavelength as compared to [Co(en)(NH3)4]3+ [Adv. 2018] A. Total number of valence shell electrons at metal centre in Fe(CO)5 or Ni(CO)4 is 16 B. These are predominantly low spin in nature. C. Metal-carbon bond strengthens when the oxidation state of the metal is lowered D. The carbonyl C-O bond weakens when the oxidation state of the metal is increased [Adv. 2017] A. Addition of silver nitrate to y gives only two equivalents of silver chloride. B. The hybridization of the central metal ion in Y is d2sp3 B. Z is a tetrahedral complex B. When X and Z are in equilibrium at 0oC, the colour of the solution is pink [Main Jan. 11, 2019 (I)] [Adv. 2018] A. A-t ; B-s, w ; C-q, r ; D-p B. A-t , wc; B-s ; C - q ; D-p, q C. A-w ; B-s, t ; C-p; D-q, r D. A-s, w ; B-t, w ; C-p, q ; D-r [Adv. 2014] A. (s) (q) (r) (p) B. (r) (p) (s) (q) C. (q) (p) (r) (s) D. (p) (r) (s) (q) Join with us in Telegram Telegram Channel ● t.me/unacademyatoms Complete Notes and Lectures ● livedaily.me/atoms India’s BEST Educators If you want to be the BEST “Learn” from the BEST Unacademy Subscription SAKSHI SAKSHI Study Planner Personal Guidance Customized study plan with bi-weekly reviews Get one on one guidance from top exam experts Live Classes ICONIC Weekly Tests PLUS Structured Courses Unlimite d Access Test Analysis Get one on one guidance from top exam experts Study Material Specialised Notes & Practice Sets Experts' Guidelines Study booster workshops by exam experts SAKSHI