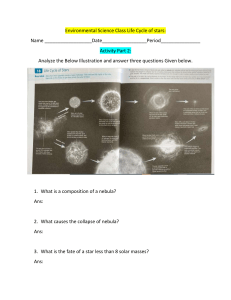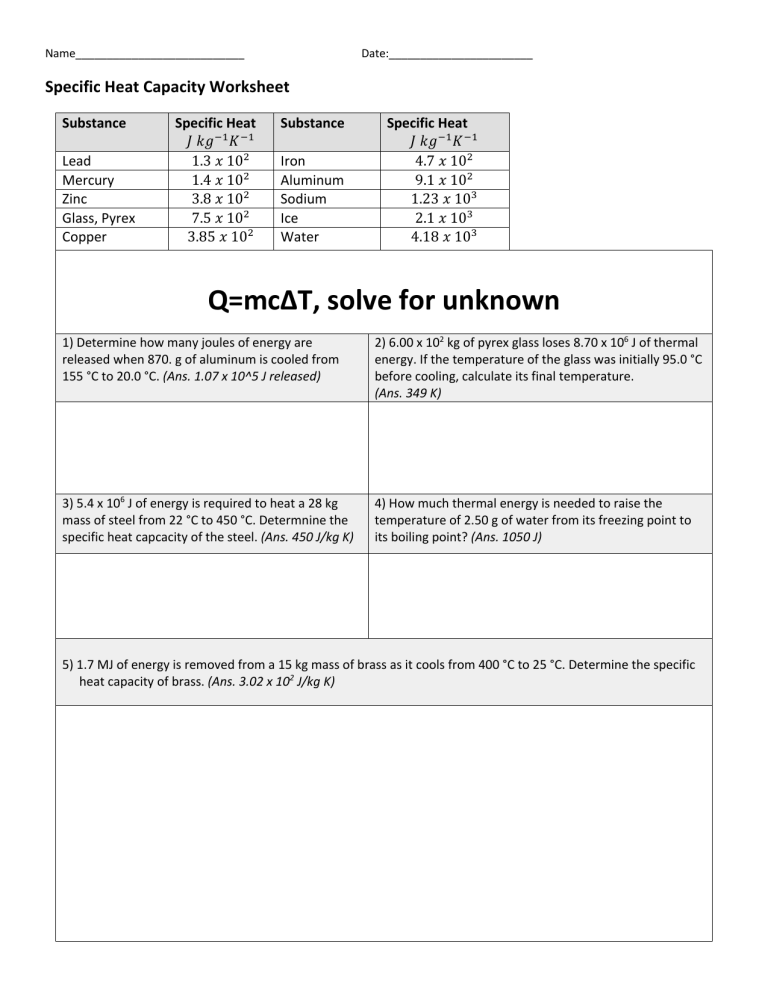
Name___________________________ Date:_______________________ Specific Heat Capacity Worksheet Substance Lead Mercury Zinc Glass, Pyrex Copper Specific Heat 𝐽 𝑘𝑔!" 𝐾 !" 1.3 𝑥 10# 1.4 𝑥 10# 3.8 𝑥 10# 7.5 𝑥 10# 3.85 𝑥 10# Substance Iron Aluminum Sodium Ice Water Specific Heat 𝐽 𝑘𝑔!" 𝐾 !" 4.7 𝑥 10# 9.1 𝑥 10# 1.23 𝑥 10$ 2.1 𝑥 10$ 4.18 𝑥 10$ Q=mcΔT, solve for unknown 1) Determine how many joules of energy are released when 870. g of aluminum is cooled from 155 °C to 20.0 °C. (Ans. 1.07 x 10^5 J released) 2) 6.00 x 102 kg of pyrex glass loses 8.70 x 106 J of thermal energy. If the temperature of the glass was initially 95.0 °C before cooling, calculate its final temperature. (Ans. 349 K) 3) 5.4 x 106 J of energy is required to heat a 28 kg mass of steel from 22 °C to 450 °C. Determnine the specific heat capcacity of the steel. (Ans. 450 J/kg K) 4) How much thermal energy is needed to raise the temperature of 2.50 g of water from its freezing point to its boiling point? (Ans. 1050 J) 5) 1.7 MJ of energy is removed from a 15 kg mass of brass as it cools from 400 °C to 25 °C. Determine the specific heat capacity of brass. (Ans. 3.02 x 102 J/kg K)