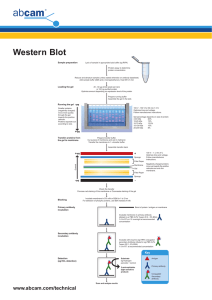
WESTERN BLOTTING Good afternoon Dr. Villordon and classmates. I am Chonnie Mae Pis-an and I will be discussing Western Blotting. To get us started, I will be defining the terms "blotting" and "SDS-PAGE". Blotting refers to the transfer of biological samples from a gel to a membrane and their subsequent detection on the surface of the membrane. Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is commonly used to obtain high resolution separation of complex mixtures of proteins. The method initially denatures the proteins that will undergo electrophoresis. Aside from Western Blotting, other "blot protocols" also exist such as the Northern and Southern Blot. The table shows their similarities and differences. [ayaw na basaha ning table, daritso na aning paragraphs] Southern, northern, and western blot protocols are similar, and begin with electrophoretic separation of protein and nucleic acid fragments on a gel, which are then transferred to a membrane (nitrocellulose membrane, polyvinylidene difluoride (PVDF) membrane, etc.) where they are immobilized. This enables radiolabeled or enzymatically labeled antibody or DNA probes to bind the immobilized target, and the molecules of interest may then be visualized with various methods. Blotting techniques are selected based on the target molecule: DNA, RNA, or protein. In summary, the three blot protocols have different target molecules and sample preparations. The Southern, Northern and Western blot have the same separation technique which is Electrophoresis. Western blot differs from both the Southern and Northern protocol in terms of membrane material. Now, let us redirect our attention to Western Blotting. DEFINITION Western blotting a well-established analytical technique for detecting, analyzing, and quantifying a specific protein molecule from among a mixture of proteins associated with a particular tissue or cell type. Western blotting typically involves protein separation by gel electrophoresis followed by transfer to a polyvinylidene difluoride (PVDF) or nitrocellulose membrane. After proteins have been transferred, they can be stained for visualization and directly identified by N-terminal sequencing, mass spectrometry or immunodetection. The membrane is exposed to an antibody specific to the target protein. Binding of the antibody is detected using a radioactive or chemical tag. This procedure was named for its similarity to the previously invented method known as the Southern blot. PURPOSE Western blot General Purposes widely used to detect specific protein molecules in complex samples such as tissue homogenates and cell lysates. sometimes used to diagnose disease. can also be used to evaluate the size of a protein of interest, and to measure the amount of protein expression. Specific Purposes The technique enables evaluation of: [only read headings] Protein–DNA interactions – Attachment of DNA binding proteins to specific DNA sequences is vital to the process of transcriptional regulation. Southwestern blotting (similar to western blotting except the membrane is probed with DNA) can be used to identify transcription factors in gene regulation studies. Protein–protein interactions – These are vital for many essential cellular processes. Detection of protein–protein interactions, using a variation of western blotting known as far-western blotting, can help to elucidate cellular function and dysfunction. Post-translational modifications (PTMs) – PTM’s impact protein folding and consequently function, expanding proteome diversity. However, aberrant PTM’s have been associated with disease. Consequently, their study is of great interest to researchers and western blot provides one such tool with which to do this. Protein isoform detection – Proteins may be expressed in differing isoforms in different cellular states with varying activities or targets. Alternatively, they may require cleavage to become activated. Antibody characterization – When antibodies are produced as tools or therapeutics, their validation and characterization is vital to ensure correct performance and safety. As an antibody-based method, western blotting is a useful technique in this process. Epitope mapping – Understanding how and where antibodies bind their target protein is valuable for research, diagnostic and therapeutic purposes. There are many tools used towards this goal and thanks to its specificity, western blotting is one. Subcellular protein localization – Performing western blot analysis of different cellular fractions allows the location of target proteins in the cell to be determined. Single-cell western blotting has offered great insights in this field and overcomes some of the antibody cross-reactivity issues experienced by other single-cell assays. BACKGROUND / HISTORY Western blot experiment, or western blotting was developed in 1979 by Harry Towbin and his colleagues and they named it “western blot” due to the technique’s similarity to Southern blotting. It is now a routine technique for protein analysis. It is also called immunoblotting because an antibody is used to specifically detect its antigen. This antibody-based technique is used to detect the presence, size and abundance of specific proteins within a sample. Western blotting can produce qualitative and semiquantitative data about the protein of interest. WESTERN BLOT PROTOCOL Overview For a successful Western blot, four requirements must be met: [do not read the bullets, go directly to paragraphs] Elution from the gel Adsorption to the membrane Retention during processing Accessibility during processing First, the protein must elute from the gel during transfer. If it is retained in the gel, it will not be available for analysis on the blot. Next, the protein must adsorb to the membrane during the transfer process. If the protein is not adsorbed, it will not be available for analysis on the blot. Third, the protein must remain adsorbed to the membrane during post transfer processing of the blot. Lastly, the adsorbed protein must be available for antibody binding. If the protein is masked, it cannot be detected. WESTERN BLOTTING STEPS IN A NUTSHELL Western blotting typically consists of three main steps such as: 1. Resolution/ Separation of a complex protein sample in a polyacrylamide gel 2. Transfer of the resolved proteins onto a membrane 3. Identification of a specific protein on the membrane But we will be breaking down these steps into a total of six procedures for better understanding. These are based on the Western Blotting Handbooks provided by the websites, ThermoFisher and Merck. SPECIFIC STEPS IN WESTERN BLOTTING 1. Electrophoretic separation of proteins Gel electrophoresis is a technique in which charged molecules, such as protein or DNA, are separated according to physical properties as they are forced through a gel by an electrical current. Proteins are commonly separated using polyacrylamide gel electrophoresis (PAGE) to characterize individual proteins in a complex sample or to examine multiple proteins within a single sample. When combined with western blotting, PAGE is a powerful analytical tool providing information on the mass, charge, purity or presence of a protein. Several forms of PAGE exist and can offer different types of information about the protein(s) of interest. Several buffering systems or gel chemistries are available for protein gel electrophoresis. Each system provides unique advantages when resolving proteins of different molecular weights. Separation of Complex Protein Mixtures in 1-D or 2-D Gels One-dimensional (1-D) sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) is commonly used to separate proteins by molecular weight prior to blotting (Figure 4). In some cases, non-denaturing conditions are used to separate native proteins. Although this method usually lacks the resolution of denaturing electrophoresis, it may be useful when the primary antibody only recognizes non-denatured proteins or when the protein’s biological activity must be retained. Two-dimensional (2-D) gel electrophoresis is the technique of choice for analyzing protein composition of cell types, tissues and fluids, and is a key technology in proteomics (Figure 5). Immunoblotting of 2-D gels provides information on molecular weight and isoelectric point and can be useful to discriminate protein isoforms generated by post-translational modifications. In some cases, protein phenotyping can be achieved by immunoblotting after only a 1-D separation by isoelectrofocusing Molecular Weight Markers The inclusion of molecular weight (MW) standards, or markers, facilitates the estimation of the sizes of the proteins of interest after resolution by electrophoresis. Two types are available, unstained and pre-stained. Unstained MW markers usually consist of a mixture of purified native or recombinant proteins of defined molecular weights. Visualizing their location on a gel or membrane requires a staining step. Pre-stained markers o allow monitoring of protein separation in the gel during electrophoresis and indicate transfer efficiency in subsequent blotting steps. o However, they can be relatively expensive and the addition of dyes may affect protein mobility. o may be less accurate for molecular weight determination, as dyes attached to the proteins may alter their ability to adsorb to the membrane during blotting. Polyacrylamide Concentration Polyacrylamide concentration can be homogenous throughout the gel or a gradient. The most common polyacrylamide concentration, 10%, is best suited for the separation of proteins in the range of 10 – 150 kDa. If unknown proteins are being analyzed or a broader range of separation is desired, gradient gels are recommended. For example, 4 – 12% Trisglycine gels are suitable for proteins in the range of 30 to 200 kDa, while 10 – 20% gels will successfully separate proteins from 6 to 150 kDa. SDS-PAGE gels are usually 1.0 and 1.5 mm thick; however, for blotting and proteins transfer best out of thinner gels (= 1 mm). Gel Running Buffers Gel running buffers are typically composed of trisglycine or tris-tricine and may contain 0.1% detergent, usually SDS. Tris-glycine buffer systems are useful for separating a wide range of protein molecular weights (6 – 200 kDa) and are compatible with denaturing or non-denaturing conditions. Tris-tricine systems are best for the separation of smaller proteins (< 10 kDa) that need to be reduced and denatured prior to loading. Both buffer systems are compatible with protein transfer to PVDF membranes. 2. Transferring proteins to a membrane Following electrophoresis, the protein must be transferred from the gel to a membrane. There are a variety of methods that have been used for this process that include, but are not limited to, diffusion transfer, capillary transfer, vacuum blotting transfer, and electroelution. The transfer method that is most commonly used for proteins is electroelution or electrophoretic transfer because of its speed and transfer efficiency. Protein transfer from gel to membrane is necessary for two reasons: better handling capability offered by the membrane than the fragile gel during western blot processing, and better target accessibility on the membrane by macromolecules like antibodies Transfer of Proteins from Gel to Membrane So, in simpler terms, the process of transferring proteins from a gel to a membrane while maintaining their relative position and resolution is known as blotting. Blotting can be achieved in three different ways: 1) Simple diffusion is accomplished by laying a membrane on top of the gel with a stack of dry filter paper on top of the membrane, and placing a weight on top of the filter paper to facilitate the diffusion process3. This method can be used to transfer proteins from one gel to multiple membranes, obtaining several imprints of the same gel. The major disadvantage of the diffusion method is that transfer is not quantitative and only transfers 25 – 50% of the proteins, compared to electroblotting. 2) Vacuum-assisted solvent flow uses the suction power of a pump to draw separated proteins from the gel onto the membrane. Both high and low molecular weight proteins can be transferred by this method; however, a smaller pore size membrane (0.2 µm) may be needed for proteins with MW < 20 kDa, since they are less readily adsorbed by the 0.45 µm membrane3. Vacuum blotting of proteins out of polyacrylamide gels is uncommon and is mostly used for nucleic acid transfer from agarose gels. 3) Electrotransfer Techniques Electrophoretic elution, or electrotransfer17 is the most commonly used transfer method in protein blotting. The principal advantages are the speed and completeness of transfer compared to diffusion or vacuum blotting. The two commonly used electrotransfer techniques are tank transfer and semi-dry transfer. Both are based on the same principles and differ only in the mechanical devices used to hold the gel/membrane stack and application of the electrical field. In tank transfer, (Figure 6) the gel/membrane stack is completely immersed in a buffer reservoir and current is applied. Typically run at constant voltage, mixing the buffer during tank transfer typically keeps the current relatively constant. While this method is effective, tank transfer is a slow technique that requires large volumes of buffer. Transfer systems for western blotting Wet transfer (tank transfer) In wet transfer, transfer efficiencies are better for lower molecular weight proteins than higher molecular weight proteins, with typical efficiencies of 80–100% for proteins between 14 and 116 kDa [2]. The transfer efficiency improves with increased transfer time. However, with increasing time and the use of membranes with larger pore sizes (0.45 µm), the risk of transferring the proteins completely through the membrane increases (also known as blow-through), especially for lower molecular weight (<30 kDa) proteins. Semi-dry transfer Semi-dry transfer became available as the need for faster results became an issue for researchers. For semi-dry protein transfer, the transfer sandwich is placed horizontally between two plate electrodes in a semi-dry transfer apparatus (Figure 5). The key to improving the speed of transfer with this method is to maximize the current passing through the gel versus around it. To do this, the amount of buffer used in the transfer is limited to that contained in the transfer sandwich. Hence, it is critical that the membrane and filter paper sheets are cut to the gel size without overhang and that the gel and filter paper are thoroughly equilibrated in transfer buffer. Also, the use of extra-thick filter paper (approximately 3 mm thickness) is helpful in certain semi-dry transfer devices because these sheets can hold more transfer buffer. Methanol may be included in the transfer buffer, but other organic solvents, including aromatic hydrocarbons, chlorinated hydrocarbons, and acetone, should not be added to avoid damage to the electrode plates. Fast-blotting, semi-dry techniques use higher ionic strength transfer buffers and a high current power supply to decrease transfer times to under 10 minutes. In rapid methods, amperage is held constant and voltage is limited to a maximum of 25 V. Transfer with traditional Towbin buffers can be preformed in a semi-dry apparatus either at constant current (0.1 up to approximately 0.4 A) or voltage (10 to 25 V) for 30 to 60 minutes. Dry transfer Dry transfer methods use a transfer sandwich containing innovative components that eliminate use of traditional transfer buffers. A unique gel matrix (transfer stack) that incorporates buffer is used instead of buffer tanks or soaked filter papers (Figure 8). The high ionic density in the gel matrix enables rapid protein transfer. During dry blotting the copper anode does not generate oxygen as a result of water electrolysis, unlike in wet and semi-dry techniques. This absence of oxygen generation reduces blot distortion. Typically, transfer time is reduced by the shortened distance between electrodes, high field strength, and high current. As dry blotting does not require the setup time of wet or semi-dry transfer, not only is the speed of transfer a major benefit, but the overall time investment is improved. 3. Blocking nonspecific sites The membrane supports used in western blotting have a high affinity for proteins. Therefore, after the transfer of the proteins from the gel, it is important to block the remaining surface of the membrane to prevent nonspecific binding of the detection antibodies during subsequent steps. A variety of blocking buffers ranging from milk or normal serum to highly purified proteins have been used to block free sites on a membrane. The blocking buffer should improve the sensitivity of the assay by reducing background interference and improving the signal-to-noise ratio. No single blocking agent is ideal for every experiment since each antibody-antigen pair has unique characteristics. Empirical testing of blocking buffers is essential in optimizing a western blot experiment. Frequently blocking buffers are made by researchers in the laboratory; however, commercially available blocking buffers offer convenience. 4. Wash buffer formulations Like other immunoassay procedures, western blotting consists of a series of incubations with different immunochemical reagents separated by wash steps. Washing steps are necessary to remove unbound reagents and reduce background, thereby increasing the signal-to-noise ratio. Insufficient washing may result in high background, while excessive washing may result in decreased sensitivity caused by elution of the antibody and/or antigen from the blot. As with other steps in western blotting blot, a variety of buffers may be used. Tris-buffered saline (TBS) and phosphate-buffered saline (PBS) are the most commonly used wash buffers. In most cases, PBS and TBS solutions can be interchangeable. However, there are situations on when to use one over the other. For example, TBS should be used when using systems with alkaline phosphatase (AP)-conjugated secondary antibodies or when detecting phosphorylated proteins with phospo-specific antibodies. Occasionally, wash buffer formulations consist of a detergent such as 0.05% Tween 20 to aid in the removal of nonspecifically-bound material. Depending on the specifics of the assay, the amount of detergent in the wash buffer will vary, though typical concentrations are from 0.05 to 0.5% for detergents like Tween 20. Another common technique is to add a 1:10 dilution of the blocking solution to the wash buffer. Including the blocking agent with the detergent may help to minimize background in the assay by preventing elution of the blocking protein from the membrane and/or allowing nonspecific interactions to occur with the protein in solution rather than those immobilized on the membrane. It is important to note that detergents, like the protein solutions, can promote microbial growth. While it is convenient to make pre-diluted stocks of detergents like NP-40, CHAPS, and Tween 20, fungi can grow in these solutions, which can lead to high background noise. In addition, detergents can contain significant amounts of peroxides which will cause background signal when using horseradish peroxidase substrates. Therefore, it is important to use high-purity detergents. 5. Primary and secondary antibodies Western blotting is typically performed by probing the blocked membrane with a primary antibody that recognizes a specific protein or epitope on a group of proteins (e.g., SH2 domain or phosphorylated tyrosine). The choice of a primary antibody for a western blot will depend on the antigen to be detected and what antibodies are available to that antigen. It is also important to note that not all primary antibodies are suitable for western blotting and the application should be verified, if possible, before purchasing a new primary antibody. In general, the primary antibody that recognizes the target protein in a western blot is not directly detectable. Therefore, tagged secondary antibodies are used as the means of ultimately detecting the target antigen (indirect detection). A wide variety of labeled secondary antibodies can be used for western blot detection. The choice of secondary antibody depends on either the species of animal in which the primary antibody was raised (the host species) or any tag linked to the primary antibody (e.g., biotin, histidine (His), hemagglutinin (HA), etc.) For example, if the primary antibody is an unmodified mouse monoclonal antibody, then the secondary antibody must be an anti-mouse IgG secondary (or non-IgG) antibody obtained from a non-mouse host. Antibodies for western blotting are typically used as dilute solutions, and manufacturers may recommend using ranges from a 1/100–1/500,000 dilution from a 1 mg/mL stock solution. However, the optimal dilution of a given antibody with a particular detection system must be determined experimentally. More sensitive detection systems require less antibody than lower sensitivity systems and can result in substantial savings on antibody costs and allow a limited supply of antibody to be stretched out over more experiments. Using lower amounts of antibody can also have the added benefit of reduced background because the limited amount of antibody shows increased specificity for the target with the highest affinity. Antibody dilutions are typically made in the wash buffer. The presence of detergent and a small amount of the blocking agent in the antibody diluent often helps to minimize background, thereby increasing the signal-to-noise ratio. Conversely, adding too much blocking agent or detergent to the antibody dilution solution can prevent efficient binding of the antibody to the antigen, causing reduced signal as well as reduced background. 6. Detection methods While there are many different tags that can be conjugated to a secondary or primary antibody, the detection method used will limit the choice of what can be used in a western blotting assay. Radioisotopes were used extensively in the past, but they are expensive, have a short shelf-life, offer no improvement in signal-to-noise ratio and require special handling and disposal. Alternative labels are enzymes and fluorophores. Enzymatic labels are most commonly used for western blotting and, although they require extra steps, can be extremely sensitive when optimized with an appropriate substrate. Horseradish peroxidase (HRP), and to a lesser extent, alkaline phosphatase (AP) are the two enzymes used most extensively as labels for protein detection. An array of chromogenic, fluorogenic, and chemiluminescent substrates are available for use with either enzyme. Alkaline phosphatase offers a distinct advantage over other enzymes in that its reaction rate remains linear, improving sensitivity by simply allowing a reaction to proceed for a longer time period. Unfortunately, the increased reaction time often leads to high background signal resulting in low signal-to-noise ratios. Horseradish peroxidase–conjugated antibodies are considered superior to antibody-AP conjugates with respect to the specific activities of both the enzyme and antibody due the smaller size of HRP enzyme and compatibility with conjugation reactions. In addition, the high activity rate, good stability, low cost, and wide availability of substrates make HRP the enzyme of choice for most applications. Enzyme-conjugated antibodies offer the most flexibility in detection and documentation methods for western blotting because of the variety of substrates available. The simplest detection/documentation system is to use chromogenic substrates. While not as sensitive as other substrates, chromogenic substrates allow direct visualization of signal development. Unfortunately, chromogenic substrates tend to fade as the blot dries or during storage, making the blot itself an unreliable means of documentation. However, it is fairly straightforward to either photocopy or directly scan the blot in order to make a permanent replica of chromogenic western blot results. Chemiluminescent blotting substrates differ from other substrates in that the signal is a transient product of the enzyme-substrate reaction and persists only as long as the reaction is occurring. If either the substrate is used up or the enzyme loses activity, then the reaction will cease and signal will be lost. However, in well-optimized assays using proper antibody dilutions and sufficient substrate, the reaction can produce stable output of light for 1 to 24 hours depending on the substrate, allowing consistent and sensitive detection that may be documented with X-ray film or digital imaging equipment. While X-ray film can be used to obtain semi-quantitative data, digital imaging is more sensitive because of the broad dynamic range of detection, allowing researchers to obtain quantitative data from western blots. The use of fluorophore-conjugated antibodies requires fewer steps because there is no substrate development step in the assay. While the protocol is shorter, this method requires special equipment in order to detect and document the fluorescent signal due to the need for an excitation light source. Recent advances in digital imaging and the development of newer generation fluorophores such as infrared, near-infrared, and quantum dots has increased the sensitivity and popularity of using fluorescent probes for western blotting and other immunoassays. Although the equipment and fluorophore-conjugated antibodies can be quite expensive, this method has the added advantage of multiplex compatibility (using more than one fluorophore in the same experiment). In addition, chemical waste is further reduced compared to other blotting procedures. Western Blot Detection Considerations Western blots detect specific protein from cells or tissues in a convenient, flexible format for rapid evaluation. The western blot format can also be quantitative and offer a high degree of sensitivity. With a variety of detection techniques, including chemiluminescent, fluorescent, or chromogenic to choose from, you can select a technology to match your experimental requirements and the instruments you have available. We discuss below a few key factors to consider before performing western blotting. Signal-to-noise ratio Signal-to-noise ratio compares the level of desired or relevant signal to the level of background noise or irrelevant signal; the higher the ratio, the better the result. In western blotting, the signal is the density of the specific probed protein band of interest; the noise is the density of the background. In western blotting applications, optimization of the signal-to-noise ratio is often more important than increasing the sensitivity of the system. The sensitivity of the system is irrelevant if the signal cannot be adequately distinguished from the noise. For information on western blot optimization methods, see page 80. Direct vs. indirect detection The antibody that recognizes a target protein is called the primary antibody. If this antibody is labeled with a tag for visualization purposes (typically an enzyme or fluorophore), direct detection of the target is possible. Typically, the primary antibody is not labeled for direct detection. Instead a secondary antibody that has been labeled with a detectable tag is used to probe for the primary antibody, which is bound to the target. Thus, the target is detected indirectly. Indirect detection with secondary antibodies requires more steps than direct detection, but it can also offer significant advantages over using primary antibodies that are directly labeled (Figure 13). Indirect methods can offer increased sensitivity through the signal amplification that occurs as multiple secondary antibody molecules bind to a single primary antibody. In addition, a given secondary antibody will recognize most primary antibodies of the same isotype and target species, making it a more versatile reagent than individually labeled primary antibodies. Several variants of these probing and detection strategies exist. However, each variant depends on a specific probe (e.g., a primary antibody) whose presence is linked directly or indirectly to some sort of measurable tag. In this handbook, most methods discussed use indirect detection, as this has emerged as the most popular detection strategy. Manual vs. automated western blot processing Traditionally, probing a western blot prior to data visualization involved a series of manual steps, many of which were individually short but collectively required significant hands-on time. Today, instruments are available to automate some of these tasks, tremendously decreasing hands-on time. Manual and automated procedures share three essential steps: blocking the membrane, probing with primary and secondary antibodies, and washing the membrane