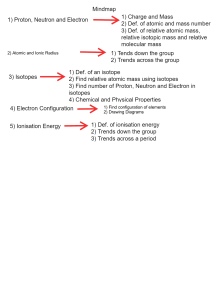
Atomic radius By Gerardo Munguia Natalia duran and Diego Atomic radios relation with the periodic table Atomic radius The atomic radius Will determine the size of the table since since if you go sownwards of the periodic table you Will find that the atomic size gets bigger and of you go to rigth atomic size gets potencially smaller This happens since every element in the rigth has one more proton in the nucleus than the last,wich means that there is a stronger electromagnetic atraction felt by the electron and the radius shrinks How to calculate atomic radius ½ of that distance is atomic radius 0 bonded atoms 0 0=nucleus ? Atomic radius is ONE HALF of the dustance between the nucleus of two bonding atoms Atomic radius represented LI Bohr diagram 55% Bohr diagrams works by levels each level can hold a set amount of electrons P1=2 P2=8 P3=8 The force of the charge is going to pull those energy levels closer to itself LITHIUM Charge is 3+ NE 45% NEON Charge is 10+ Chemistry Lesson Infographics Mars Despite being red, Mars is a very cold place 01 Neptune 03 Earth 05 Neptune is the farthest planet from the Sun Earth is the planet where we all live on 02 07 Venus https://www.youtube.c om/watch?v=4Url0pjbc uU&t=26s 04 06 Mercury Mercury is the closest planet to the Sun Jupiter Jupiter is the biggest planet of them all Saturn Saturn is the ringed one. It’s a gas giant The end!!!!