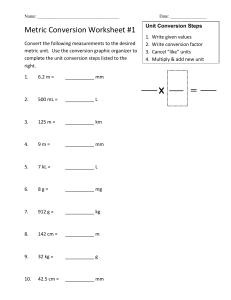
Introduction to Inorganic Chemistry FOUNDATIONS OF CHEMISTRY 1 (Inorganic Chemistry ) A Learning package for BSMB 1 Mariel Ann B. Corpuz Introduction to Inorganic Chemistry Unit 2 Measurement – a tool in Chemistry A unit of measurement is a definite magnitude of a quantity, defined and adopted by convention or by law, in other words, everyone agrees that it is used as a standard for measurement of a particular physical quantity. Any other quantity of that kind can be expressed as a multiple of the unit of measurement. A metre describes a length, a kilogram describes mass, a second describes time and etc. The metric system originates back to the 1700s in France. It is known as a decimal system because conversions between units are based on powers of ten. This is quite different to the Imperial system of units where every conversion has a unique value. In this chapter you will learn about the different types of units and conversions, its importance in our everyday lives and how it helped us in solving mathematical and chemistry problems. I. . Learning Outcomes At the end of this unit, you are expected to: 1. Define and classify the different systems of measurement. 2. Understand the principle of converting Metric-Metric units, English-English units, English -Metric units and vice versa. 3. Apply the principles of conversion of units in solving real world problems. II. Pretest 1. A metric unit for weight a. kg b. m c. degree Celsius d. ft 2. The degree or intensity of heat present in a substance or object a. Density b. Temperature c. Length d. Humidity 3. The space occupied by a flat shape or the surface of an object a. Matter b. Mass c. Area d. Volume Introduction to Inorganic Chemistry III. Content Measurement Magnitudes of measurements are typically given in terms of a specific unit. The two systems used for specifying units of measure are the English and Metric systems. Units in the English system are historical units of measurement used in medieval England which evolved from the AngloSaxon and Roman systems. The Metric system is a decimalized system of measurement developed in France in late 18th century. Since the metric system is almost universally used, it is often referred to as the International System of Units and abbreviated SI. The Metric System The metric system is the preferred system of scientific units for several reasons: The majority of countries in the world employ the metric system of measurement. The prefixes attached to metric units carry the same meaning for all base units. The metric system is based upon powers of ten, which is convenient because: 1. A measurement in the metric system that is represented by a rational number remains a rational number after metric unit conversion. (For example, 250 mm = 25 cm = 0.25 m). In contrast irrational unit systems, such as the English system, do not have the same property (For example, 250 inches = 20.8333... ft = 0.0039457... mile) 2. Because metric units are decimal-based, they are easily converted by moving the decimal point. Table 1: SI Base Units Introduction to Inorganic Chemistry Table 2: Some examples of SI derived units Table 3: SI derived units with SI based unit equivalents If units are named after a person, then a capital letter is used for the first letter. Often, liters is written with a capital (L) because a lowercase (l) looks like a one (1). An important feature of the metric system is the use of prefixes to express larger and smaller values of a quantity. For example, a large number of grams can be expressed in kilograms, and a fraction of a gram could be expressed in milligrams. Commonly used prefixes are listed in Table 4 below. Introduction to Inorganic Chemistry Table 4: Metric Prefixes Using prefixes, conversions between units can be devised. For example: 1kg = 1000g on the left-hand side the prefix is used. On the right-hand side, the prefix is replaced with the multiplication factor. 1mg = 0.001g on the left-hand side the prefix is used. On the right-hand side, the prefix is replaced with the multiplication factor. To make the conversion friendlier to use, multiply both sides by 1000 (Why 1000? Because milli means one thousandth and one thousand thousandths make one whole), so 1000mg = 1g. 1Mm = 1 000 000m on the left-hand side the prefix is used. On the right-hand side, the prefix is replaced with the multiplication factor. 1μm = 0.000 001m on the left-hand side the prefix is used. On the right-hand side, the prefix is replaced with the multiplication factor. To make the conversion friendlier to use, multiply both sides by 1 000 000 (Why 1 000 000? Because micro means one millionth), so 1 000 000μm = 1 m Introduction to Inorganic Chemistry In the metric system, each basic type of measurement (length, weight, capacity) has one basic unit of measure (meter, gram, liter). Conversions are quickly made by multiplying or dividing by factors of 10. It is as simple as moving the decimal point to the right (for smaller prefixes) or to the left (for larger prefixes). The prefixes instantly convey how many decimal place moves are required of the numeric value. For example, using the scientific notation value of the metric prefixes: 1 cm = 1 centimeter = 1 × 10-2 meter = 0.01 meter 1 kg = 1 kilogram = 1 × 103 gram = 1000 gram 1 µL= 1 microliter = 1 × 10-6 liter = 0.000001 liter In addition to the standard metric prefixes, the Ångstrom is also a commonly used unit that arises in measurements on the atomic scale: 1 Ångstrom = 1 Å = 1 × 10-10 meter. Metric-Metric Conversion of Units 1. Mass The base metric unit for mass is the gram. Mass is the correct term for what is commonly called weight. On Earth, there is no difference in the value of mass and weight. Table 5: Unit Conversion for Mass Examples: 1. change 4500g to kg When changing from a smaller unit (g) to a larger unit (kg), a smaller value will be the result. The conversion involves grams and kilograms, so the conversion required is 1000g = 1kg. Using the dimensional analysis method, 4500𝑔 × 1𝑘𝑔 = 4.5𝑘𝑔 1000𝑔 Introduction to Inorganic Chemistry 2. change 3.25t to kg When changing from a larger unit (g) to a smaller unit (kg), a larger value will be the result. The conversion involves tonnes and kilograms, so the conversion required is 1000t = 1kg. Using the dimensional analysis method, 3.25𝑡 × 3. change 1.2g to mg 1000𝑘𝑔 = 3,250𝑘𝑔 1𝑡 Using the table above, the conversion from grams to milligrams requires multiplying by 1000. Using the dimensional analysis method, 1.2𝑔 × 1000𝑚𝑔 = 1,200𝑚𝑔 1𝑔 2. Length The base metric unit for length is the metre. Length is the same as distance. For most quantities, conversions are usually based on 1000. Table 6: Unit Conversions for Length Examples: 1. change 7900m to km When changing from a smaller unit (m) to a larger unit (km), a smaller value will be the result. The conversion involves meters and kilometers, so the conversion required is1000m = 1km. Introduction to Inorganic Chemistry Using the dimensional analysis method, 7,900𝑚 × 1𝑘𝑚 = 7.9𝑚 1000𝑚 2. change 0.532km to cm There is no conversion to change from km to cm. To achieve this, two conversions are required, they are, km to m and then m to cm. 0.532 km to meters uses the conversion multiplying by 1000. (Based on the table above). Using the dimensional analysis method, 0.532𝑘𝑚 × 1000𝑚 100𝑐𝑚 × = 53,200𝑐𝑚 𝑘𝑚 1𝑚 3. Capacity The base metric unit for capacity is Liters. Capacity is how much a container can hold or is holding with particular reference to fluid. Closely related to this is the concept of volume which is the amount of space within a container. Table 7: Unit Conversion for Capacity Examples: 1. change 10350L to kL When changing from a smaller unit (L) to a larger unit (kL), a smaller value will be the result. The conversion involves metres and kilometres, so the conversion required is1000L = kL. Using the dimensional analysis method, 10,350𝐿 × 1𝑘𝐿 = 10.35𝑘𝐿 1000𝐿 Introduction to Inorganic Chemistry 2. 3kL to mL There is no conversion to change from kL to mL. To achieve this, two conversions are required, they are, kL to L and then L to kL. Using the dimensional analysis method, 3𝑘𝐿 × 1000𝐿 1000𝑚𝐿 × = 3,000,000𝑚𝐿 𝑜𝑟 3.0 × 106 𝑚𝐿 1𝑘𝐿 1𝐿 4. Area The base unit for area is square meters 2m. A square metre is a square with side length 1 metre. A square centimeter is a square with side length 1 centimeter. Conversions are required the change between square centimeters, square meters, hectares and square kilometers. Area unit conversions can be derived from length unit conversions. Using the length conversion100cm = 1m, the area unit conversions can be obtained by squaring everything in the conversion; Similarly, using the length conversion 10001mkm=, the area unit conversions can be obtained by squaring everything in the conversion; Hectares are a unit of area that is in between a square metre and a square kilometer. Examples: 1. Change 45,000m2 to ha The conversion involves square meters and hectares, so the conversion required is 10,000m2 = ha Using the dimensional analysis method, Introduction to Inorganic Chemistry 45,000𝑚2 × 1ℎ𝑎 = 4.5ℎ𝑎 10,000𝑚2 5. Volume The base unit for volume is cubic meters m3. A cubic metre is a cube with side length 1 metre. A cubic centimeter is a cube with side length 1 centimeter. Conversions are required the change between cubic centimeters, cubic meters, and cubic kilometers. Volume unit conversions can be derived from length unit conversions. Using the length conversion 100cm =1m, the volume units can be obtained by cubing everything in the conversion, Using the length conversion 1000m =1km, the volume units can be obtained by cubing everything in the conversion, Unit conversions for volume units are in the table below. Example: 1. Change 3.15m3 to cm3 Using the dimensional analysis method, 3.15𝑚3 × (100𝑐𝑚)3 1,000,000𝑐𝑚3 3 = 3.15𝑚 × = 3, 150,000𝑐𝑚3 (1𝑚)3 1𝑚3 Introduction to Inorganic Chemistry 2. What is the capacity of the container below? The solution is, The English System While the metric system was lawfully accepted for use in the United States in 1866, the US has not adopted the metric system as its "official" system of measurement. The US English System of measurement grew out of the manner in which people secured measurements using body parts and familiar objects. A standard was then eventually set to ensure that all measurements represented the same amount for everyone. See below for the US customary system of measures. 1. Length The basic units for length or distance measurements in the English system are the inch, foot, yard, and mile. Other units of length also include the rod, furlong, and chain. Example: 1. convert 84 inches to feet Using the dimensional analysis method, 84 𝑖𝑛𝑐ℎ𝑒𝑠 × 1 𝑓𝑜𝑜𝑡 = 7𝑓𝑒𝑒𝑡 12 𝑖𝑛𝑐ℎ𝑒𝑠 2. convert 10,000 feet to miles Using the dimensional analysis method, 10,000 𝑓𝑒𝑒𝑡 × 1 𝑚𝑖𝑙𝑒 = 1.894 𝑚𝑖𝑙𝑒𝑠 5, 280 𝑓𝑒𝑒𝑡 Introduction to Inorganic Chemistry 2. Area In the English system, areas are typically given in square feet or square yards. For larger area measurements, the acre or square mile may be used. Example: 1. convert 500 square inches to square feet Using the dimensional analysis method, 500𝑖𝑛𝑐ℎ2 × 1𝑓𝑡 2 = 3.472𝑓𝑡 2 144𝑖𝑛𝑐ℎ2 2. convert 50,000 acres to square mile Using the dimensional analysis method, 1𝑚𝑖 2 50,000 𝑎𝑐𝑟𝑒𝑠 × = 78.125𝑚𝑖 2 640 𝑎𝑐𝑟𝑒𝑠 3. Volume Volumes in the English system are typically given in cubic feet or cubic yards. For larger volumes, such as the quantity of water in a reservoir, the acre-foot unit is used. It is equivalent to the area of an acre having a depth of 1 foot. Example: 1. convert 20 cubic inches to cubic feet Using the dimensional analysis method, 1𝑓𝑡 3 20 𝑖𝑛𝑐ℎ × = 11.57 𝑓𝑡 3 1, 728 𝑖𝑛𝑐ℎ3 3 Introduction to Inorganic Chemistry 2. Another solution to Example 1 is by using the equivalent 1 foot = 12 inches. Using the dimensional analysis method, 20 𝑖𝑛𝑐ℎ3 × (1 𝑓𝑜𝑜𝑡)3 (12 𝑖𝑛𝑐ℎ)3 = 20 𝑖𝑛𝑐ℎ3 × (1)3 𝑓𝑜𝑜𝑡 3 (12)3 𝑖𝑛𝑐ℎ3 = 20 𝑖𝑛𝑐ℎ3 × 1 𝑓𝑜𝑜𝑡 3 1,728 𝑖𝑛𝑐ℎ3 = 11.57 𝑓𝑡 3 4. Mass The avoirdupois pound is the primary unit of mass in the English system. Avoirdupois is a system of weight based on the 16 ounces per pound rather than the 12 ounces per pound in the troy system of weight. Example: convert 6 ton (long) to ounce Using the dimensional analysis method, 6 𝑡𝑜𝑛 × 2,240 𝑝𝑜𝑢𝑛𝑑𝑠 16 𝑜𝑢𝑛𝑐𝑒𝑠 × = 215,040 𝑜𝑢𝑛𝑐𝑒𝑠 1 𝑡𝑜𝑛 1 𝑝𝑜𝑢𝑛𝑑𝑠 1. Angular Measurement In geometry, any horizontal or vertical angle is measured in degrees. These angles may be given in decimal degrees or degrees, minutes, and seconds. The radian is another unit of measure for angles. By definition, a full circle has 2 π radians or 360 degrees. Example: Convert 190 degrees to radians 190° × 2𝜋 𝑟𝑎𝑑𝑖𝑎𝑛𝑠 = 3.316 𝑟𝑎𝑑𝑖𝑎𝑛𝑠 360° Introduction to Inorganic Chemistry Table 8: Summary of the English Customary System of Measures Introduction to Inorganic Chemistry Temperature, Pressure, Density and Specific Gravity 1. Temperature a. English Units The Fahrenheit scale, or degrees Fahrenheit (°F), is used in the United States to measure temperature. On the Fahrenheit scale, the freezing point of water is 32°F while the boiling point is 212°F at standard atmospheric pressure. The boiling and freezing points of water are exactly 180 degrees apart, making each degree Fahrenheit 1/180 of the interval between the two points. b. Metric Units The Celsius scale, or degrees Celsius (°C), is used the metric system to measure temperature. On the Celsius scale, the freezing point of water is 0°C while the boiling point is 100°C at standard atmospheric pressure. The boiling and freezing points of water are exactly 100 degrees apart, making each degree Celsius 1/100 of the interval between the two points. The Fahrenheit and Celsius scales converge at -40° (i.e. -40°F and -40°C are the same temperature). d. ° 𝐾 = ℃ + 273.15 e. °𝑅 = ℉ + 460 Example: 1. Convert 200 degrees Celsius to degrees Fahrenheit The solution is, 9 ℉ = (200°𝐶 × ) + 32 = 392℉ 5 Introduction to Inorganic Chemistry 2. Convert 360 degrees Kelvin to degrees Fahrenheit The solution is, Step 1: Convert degrees Kelvin to degrees Celsius using the formula ° 𝐾 = ℃ + 273.15 . So, ℃ = °𝐾 − 273.15 ℃ = 360 − 273.15 = 86.85 ℃ Step 2: From degrees Celsius convert to degrees Fahrenheit using the formula 9 ℉ = ( × ℃ ) + 32 5 9 ℉ = ( × 86.85) + 32 = 188.33℉ 5 2. Pressure Atmospheric pressure is the force per unit area exerted against a surface by the weight of the Earth’s atmosphere above that surface. Because there is less overlying atmospheric mass as elevation increases, pressure decreases with increasing elevation. The standard atmosphere (atm) is an international reference for pressure. a. English units In the English system, air pressure is typically measured in inches mercury (inHg). 1 𝑎𝑡𝑚 = 29.2125𝑖𝑛𝐻𝑔 b. Metric units Air pressure is measured in millimeters mercury (mmHg) or millibars (bars) in the metric system, but may also be measured in pascals or kilopascals. 1 𝑎𝑡𝑚 = 101,325 𝑃𝑎 = 1.01325 𝑏𝑎𝑟𝑠 = 760 𝑚𝑚𝐻𝑔 c. English to Metric Conversions 1 𝑖𝑛𝐻𝑔 × 1000 = 25.40 𝑚𝑚𝐻𝑔 39.37 3. Density and Specific Gravity Density and specific gravity are both used to describe mass and can be used to compare substances. However, they are not identical measures. Density is defined as mass per unit volume (m/V). It has the SI unit kg/m3 and is an absolute quantity. Density in Metric to English unit conversion is, 1 𝑘𝑔 𝑙𝑏𝑚 = 0.062 3 𝑚 𝑓𝑡 3 Introduction to Inorganic Chemistry Specific gravity is the ratio of a material's density with that of water at 4 °C (where it is most dense and is taken to have the value 999.974 kg m-3). It is therefore a relative quantity with no units. The formula for specific gravity, given that the reference substance is water, is the density of the object divided by the density of the water. 𝑆. 𝐺. = 𝜌𝑠𝑢𝑏𝑠𝑡𝑎𝑛𝑐𝑒 𝜌𝑤𝑎𝑡𝑒𝑟 Where: 𝑆. 𝐺. = 𝑠𝑝𝑒𝑐𝑖𝑓𝑖𝑐 𝑔𝑟𝑎𝑣𝑖𝑡𝑦 𝜌𝑠𝑢𝑏𝑠𝑡𝑎𝑛𝑐𝑒 = 𝑑𝑒𝑛𝑠𝑖𝑡𝑦 𝑜𝑓 𝑡ℎ𝑒 𝑠𝑢𝑏𝑠𝑡𝑎𝑛𝑐𝑒 𝑏𝑒𝑖𝑛𝑔 𝑚𝑒𝑎𝑠𝑢𝑟𝑒𝑑 𝜌𝑤𝑎𝑡𝑒𝑟 = 𝑑𝑒𝑛𝑠𝑖𝑡𝑦 𝑜𝑓 𝑡ℎ𝑒 𝑟𝑒𝑓𝑒𝑟𝑒𝑛𝑐𝑒 Examples: 1. You have a rock with a volume of 15cm3 and a mass of 45 g. What is its density in kg/m3? Using the dimensional analysis method, 𝑚 45𝑔 𝑔 (100)3 (𝑐𝑚)3 1𝑘𝑔 𝑘𝑔 𝜌= = = 3 × × = 3000 3 3 3 3 3 𝑉 15𝑐𝑚 𝑐𝑚 (1) (𝑚) 1000𝑔 𝑚 2. You have a sample of granite with density 2.8 g/cm3. The density of water is 1.0 g/cm3. What is the specific gravity of your granite? Specific gravity is the density of the substance divided by the density of water, so, Introduction to Inorganic Chemistry Table 9: English – Metric Conversion Introduction to Inorganic Chemistry Table 10: Units of Time I HOPE YOU’RE HAVING FUN READING THE CONTENTS. Now that you had learned about the introduction to chemistry, do the succeeding learning activities. If you have questions regarding the activity, you may visit our google class or message me in our group chat. You may also contact me with the contact number I provided in our learning agreement. Don’t forget to introduce yourself and give your subject/course. If you have poor connectivity, you are given another week to accomplish the tasks. Introduction to Inorganic Chemistry IV. Learning Activities Activity 1: Measurement Directions: In your own home, measure the following according to the units given. 1. 2. 3. 4. 5. The length of your dining table (in meters) Floor area of your kitchen (in square feet) Your weight in (pounds) Surface area of your study table (in square centimeter) Volume of your pail (give at least 1 – in Liters) Activity 2. Conversion Directions: Convert the following. 1. Change the following length measurements to the units shown in brackets a. 55m (km) b. 0.325feet (inch) c. 2000km (mi) 2. Change the following mass measurements to the units shown in brackets a. 0.52g (mg) b. 2,905 kg (g) c. 10,000 pounds (short ton) 3. Change the following capacity measurements to the units shown in brackets a. 8500mL (L) b. 1.6 mL (L) c. 85.9L (kL) 4. Change the following area measurements to the units shown in brackets a. 25 000m2 (ha) b. 31.8ha (km2) c. 26cm2 (m2) 5. Change the following volume measurements to the units shown in brackets a. 375 cm3 (m3) b. 2.575 m3 (cm3) c. 356 000cm3 (m3) 6. Change the following volume units to the capacity units shown in brackets a. 67 500 cm3 (kL) b. 0.072 m3 (L) c. 345 cm3 (mL) Introduction to Inorganic Chemistry Activity 3. Essay Directions: Why is it important to learn how to convert units of measurement? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ________________________________________________________________________. V. Learning Activities Test 1. Identification Directions: Answer the following. 1. What are the SI base units for length, mass and time? 2. What is difference between the prefix m and M? 3. What is the difference between volume and capacity? Test 2. Dimensional Analysis Directions: Change the following measurements using the dimensional analysis method to the units shown in brackets. 1. 8 550 g (t)) 2. 15 305mg (kg) 3. 21.8ha (m2) 4. 2 905 μg (g) 5. 9.352L (mL) Directions: Change the following metric rates to the rate shown in brackets. 1. 850mL/hr. (L/hr.) 2. 85.9km/hr. (m/min) 3. 0.000 6 cm3/sec (L/hr.) 4. 4.51L/min (L/hr.) 5. 1.6 m2/hr. (cm2/sec) Directions: Change the following metric and imperial units to the units shown, given the conversion. 1. 2. 3. 4. 5. Change 100 meters to yards (yd) given that 1 m is 1.09yd Change 100 miles to km given that 1 mile is 1.61 km Change 36.5 oz (ounces weight) to g given that 1 oz is 28.35g Change 50 lbs. (pounds weight) to kg given that 1 kg is 2.2 lbs. Change 25 ha to acres given that 1 hectare is 2.48 acres Introduction to Inorganic Chemistry Directions: Change the following rates to the new rate using both imperial and metric units, given the conversion. 1. Change 3.45 mi/hr. to km/hr. given that 1 mile is 1.61 km 2. Change 3.45 ft2/hr. to cm2/sec given that 1 ft (foot) is 30.48 cm 3. Change 6.45 gal/hr. to L/min given that 1 imp. gallon is 4.55 liters Test 3. Problem Solving (Density and Specific Gravity) Directions: Solve the following problems: 1. You have a sample of granite with density 174.8 lbs/ft3. The density of water is 62.4 lbs/ft3. What is the specific gravity of the granite now? 2. You have a piece of log with a mass of 200kg and a volume of 25m3. What is its density?