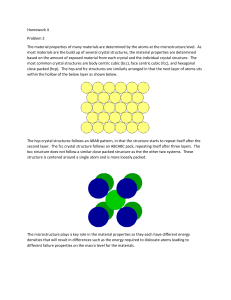
Material Engineering MEEN :2311 Structure of Crystalline Solids Structure of Crystalline Solids • How do atoms assemble into solid structures? • How does the density of a material depend on its structure? • When do material properties vary with the sample (i.e., part) orientation? 2 Materials and Packing Solid materials may be classified according to the regularity with which atoms or ions are arranged with respect to one another Crystalline materials... • atoms pack in periodic, 3D arrays • typical of: -metals -many ceramics -some polymers Noncrystalline materials... • atoms have no periodic packing • occurs for: -complex structures -rapid cooling "Amorphous" = Noncrystalline crystalline SiO2 Si Oxygen Non crystalline SiO2 3 Materials and Packing Crystalline materials... • atoms pack in periodic, 3D arrays • typical of: -metals -many ceramics -some polymers crystalline SiO2 Single crystal: atoms are in a repeating or periodic array over the entire extent of the material Polycrystalline material: comprised of many small crystals or grains 4 Crystal Structures: Energy and Packing Why do atoms assemble into ordered structures (crystals)? • Non dense, random packing Energy of interatomic bond • Dense, ordered packing Energy typical neighbor bond energy r typical neighbor bond energy Energy r Nearest neighbor atoms distances tend to be small in order to lower bond energy. Dense, ordered packed structures tend to have lower energies Crystal Structures Atoms are considered as hard spheres with well-defined radii. The shortest distance between two like atoms is one diameter of the hard sphere. We can consider crystalline structure as a lattice of points at atom/sphere centers Unit Cell The unit cell is a structural unit or building block that describe the crystal structure. Repetition of the unit cell generates the entire crystal. CRYSTAL STRUCTURES Metallic Crystal Structures Metals are usually (poly) crystalline; although formation of amorphous metals is possible by rapid cooling Atomic bonding in metals (Metallic Bonds) is non-directional ⇒ no restriction on numbers or positions of nearest-neighbor atoms ⇒ large number of nearest neighbors and dense atomic packing Atomic (hard sphere) radius, R, defined by ion core radius typically 0.1 - 0.2 nm The most common types of unit cells are faced-centered cubic (FCC) body-centered cubic (BCC) hexagonal close-packed (HCP) Face Centered Cubic Crystal Structure (FCC) Atoms are located at each of the corners and on the centers of all the faces of cubic unit cell Cu, Al, Ag, Au have this crystal structure Atoms touch each other along face diagonals Cube edge length, a ⇒ Face Centered Cubic Crystal Structure (FCC) Number of atoms per unit cell, n = 4. (For an atom that is shared with m adjacent unit cells, we only count a fraction of the atom, 1/m). In FCC unit cell we have: Each face atom(6) shared by two cells: 6×1/2 = 3 Each corner atom(8) shared by eight cells: 8×1/8 = 1 Coordination number, CN =12 Number of closest neighbors to which an atom is bonded = number of touching atoms, ABCABC... Stacking Sequence Face Centered Cubic Crystal Structure (FCC) Atomic packing factor, APF fraction of volume occupied by hard spheres = (Sum of atomic volumes)/(Volume of cell) = 0.74 (maximum possible) APF= (Sum of atomic volumes)/(Volume of unit cell) Volume of 4 hard spheres in the unit cell = 4 x 4/3 π R3 Volume of the unit cell = a3 = FCC is a close-packed structure with highest density of atoms Body Centered Cubic Crystal Structure (BCC) Atom at each corner and at center of cubic unit cell Cr, α-Fe, Mo, Tantalum have this crystal structure The coordination number, CN = 8 Number of atoms per unit cell, n = 2 Center atom (1) shared by no other cells: 1 x 1 = 1 8 corner atoms shared by eight cells: 8 x 1/8 = 1 Body Centered Cubic Crystal Structure (BCC) The hard spheres touch one another along cube diagonal 3 3a a 3 a 2 2a 2 1 a atoms R Cube diagonal length = 4R = 3 a a 4 1 a= 4R/√3 volume atom p ( 3a/4) 3 2 unit cell 3 APF = volume 3 a APF= 0.68 unit cell Hexagonal Close-Packed Crystal Structure (HCP) HCP is one more common structure of metallic crystals Six atoms form regular hexagon, surrounding one atom in center. Another plane is situated halfway up unit cell (c-axis), with 3 additional atoms situated at interstices of hexagonal (close-packed) planes Cd, Mg, Zn, Ti have this crystal structure Number of atoms per unit cell, n = 6. • 3 mid-plane atoms shared by no other cells: 3 x 1 = 3 • 12 hexagonal corner atoms shared by 6 cells: 12 x 1/6 = 2 • 2 top/bottom plane center atoms shared by 2 cells: 2 x 1/2 = 1 Unit cell has two lattice parameters a and c. Ideal ratio c/a = 1.633 Hexagonal Close-Packed Crystal Structure (HCP) The coordination number, CN =12 (same as in FCC) Atomic packing factor, APF = 0.74 (same as in FCC) a = 2R, c = 1.633a Volume of one HCP unit Cell = [Area of triangle ABC)x 6] x c Area of triangle = ½ Base x height The difference between the FCC and HCP structures is in the stacking sequence. Cubic lattice structures allow slippage to occur more easily than noncubic lattices, so HCP metals are not as ductile as the FCC metals Density, r Computation Entire crystal can be generated by the repetition of the unit cell. Density of a crystalline material, ρ Mass of total atoms in a unit cell Density = r = Total Volume of Unit Cell r = where nA VC NA n = number of atoms/unit cell A = atomic weight (g/mol) VC = Volume of unit cell (cm3)= a3 for cubic NA = Avogadro’s number = 6.022 x 1023 atoms/mol Example: Copper • crystal structure = FCC: 4 atoms/unit cell rCu = 8.89 g/cm3 • atomic weight = 63.55 g/molResult: (1 amu =theoretical 1 g/mol) • atomic radius R = 0.128 nm (1 nm = 10-7 cm) Characteristics of Selected Elements at 20°C At. Weight Element Symbol (amu) Aluminum Al 26.98 Argon Ar 39.95 Barium Ba 137.33 Beryllium Be 9.012 Boron B 10.81 Bromine Br 79.90 Cadmium Cd 112.41 Calcium Ca 40.08 Carbon C 12.011 Cesium Cs 132.91 Chlorine Cl 35.45 Chromium Cr 52.00 Cobalt Co 58.93 Copper Cu 63.55 Flourine F 19.00 Gallium Ga 69.72 Germanium Ge 72.59 Gold Au 196.97 Helium He 4.003 Hydrogen H 1.008 Density (g/cm3) 2.71 -----3.5 1.85 2.34 -----8.65 1.55 2.25 1.87 -----7.19 8.9 8.94 -----5.90 5.32 19.32 ----------- Atomic radius (nm) 0.143 -----0.217 0.114 ----------0.149 0.197 0.071 0.265 -----0.125 0.125 0.128 -----0.122 0.122 0.144 ----------12 Densities of Material Classes In general rmetals > rceramics > rpolymers Why? Polymers have... r (g/cm3 ) • close-packing (metallic bonding) • often large atomic masses • less dense packing • often lighter elements • low packing density (often amorphous) • lighter elements (C,H,O) Composites have... • intermediate values Graphite/ Ceramics/ Semicond Polymers Composites/ fibers 30 Metals have... Ceramics have... Metals/ Alloys 20 Platinum Gold, W Tantalum 10 Silver, Mo Cu,Ni Steels Tin, Zinc 5 4 3 2 1 0.5 0.4 0.3 Titanium Aluminum Magnesium *GFRE, CFRE, & AFRE are Glass, Carbon, & Aramid Fiber-Reinforced Epoxy composites (values based on 60% volume fraction of aligned fibers in an epoxy matrix). Zirconia Al oxide Diamond Si nitride Glass -soda Concrete Silicon Graphite PTFE Silicone PVC PET PC HDPE, PS PP, LDPE Glass fibers GFRE* Carbon fibers CFRE* Aramid fibers AFRE* Wood 20 CRYSTAL SYSTEMS • Unit cell = 3-dimensional unit that repeats in space • Unit cell geometry completely specified by a, b, c & a, b, g (lattice parameters or lattice constants) CRYSTALLOGRAPHIC POINTS, DIRECTIONS & PLANES • In crystalline materials, often necessary to specify points, directions and planes within unit cell and in crystal lattice • Three numbers (or indices) used to designate points, directions (lines) or planes, based on basic geometric notions • The three indices are determined by placing the origin at one of the corners of the unit cell, and the coordinate axes along the unit cell edges POINT COORDINATES Any point within a unit cell specified as fractional multiples of the unit cell edge lengths Position P specified as q r s; convention: coordinates not separated by commas or punctuation marks EXAMPLE: POINT COORDINATES • Locate the point (1/4 1 ½) • Specify point coordinates for all atom positions for a BCC unit cell – Answer: 0 0 0, 1 0 0, 1 1 0, 0 1 0, ½ ½ ½, 0 0 1, 1 0 1, 1 1 1, 0 1 1 Notation for lattice positions CRYSTALLOGRAPHIC DIRECTIONS • Defined as line between two points: a vector • Steps for finding the 3 indices denoting a direction – Determine the point positions of a beginning point (X1 Y1 Z1) and a ending point (X2 Y2 Z2) for direction, in terms of unit cell edges – Calculate difference between ending and starting point – Multiply the differences by a common constant to convert them to the smallest possible integers u, v, w – The three indices are not separated by commas and are enclosed in square brackets: [uvw] – If any of the indices is negative, a bar is placed in top of that index Notation for lattice directions COMMON DIRECTIONS EXAMPLES: DIRECTIONS Draw a [1,-1,0] direction within a cubic unit cell • Determine the indices for this direction – Answer: [120] CRYSTALLOGRAPHIC PLANES • Crystallographic planes specified by 3 Miller indices as (hkl) • Procedure for determining h, k and l: Z 1. 2. 3. 4. 5. 6. If plane passes through origin, translate plane or choose new origin Determine intercepts of planes on each of the axes in terms of unit cell edge lengths (lattice parameters) (½ ¼ ½) . Note: if plane has no intercept to an axis (i.e., it is parallel to that axis), intercept is infinity Determine reciprocal of the three intercepts (2 4 2) If necessary, multiply these three numbers by a common factor which converts all the reciprocals to small integers (1 2 1) The three indices are not separated by commas and are enclosed in curved brackets: (hkl) (121) If any of the indices is negative, a bar is placed in top of that index X 1/2 1/4 Y 1/2 (1 2 1) Crystallographic Planes example 1. Intercepts 2. Reciprocals 3. Reduction 4. Miller Indices example a b z c c b a x a b y z c c 1. 2. Intercepts Reciprocals 3. Reduction 4. Miller Indices y a b x 31 Crystallographic Planes z example 1. Intercepts 2. Reciprocals 3. Reduction a 1 1/1 1 1 4. Miller Indices (110) example 1. Intercepts 2. Reciprocals 3. Reduction a 1/2 1/½ 2 1 4. Miller Indices (100) b 1 1/1 1 1 c 1/ 0 0 c y b a x b 1/ 0 0 c 1/ 0 0 z c y a b x 32 Crystallographic Planes • Construct a (0,-1,1) plane Notation for lattice planes. THREE IMPORTANT CRYSTAL PLANES • Parallel planes are equivalent For further practice https://www.doitpoms.ac.uk/tlplib/miller_indices/lattice_examples.php Polymorphism and Allotropy Some materials (compounds) may exist in more than one crystal structure, this is called polymorphism. If the material is an elemental solid, it is called allotropy. Elements exhibiting allotropy include tin, carbon, titanium, sulfur, phosphorus, and oxygen. Carbon, which can exist as diamond, graphite, and amorphous carbon. Polymorphism and Allotropy Iron (Fe) system liquid BCC 1538ºC -Fe FCC 1394ºC g-Fe 912ºC BCC a-Fe Single Crystals & Polycrystalline Materials Single crystal: atoms are in a repeating or periodic array over the entire extent of the material Polycrystalline material: comprised of many small crystals or grains. The grains have different crystallographic orientation. There exist atomic mismatch within the regions where grains meet. These regions are called grain boundaries. Anisotropy Different directions in a crystal have different packing. For instance, atoms along the edge of FCC unit cell are more separated than along the face diagonal. This causes Anisotropy in the properties of crystals, for instance, the deformation depends on the direction in which a stress is applied In polycrystalline materials, grain orientations are random, so bulk material properties are isotropic E (diagonal) = 273 GPa E (edge) = 125 GPa Non Crystalline Amorphous Solids Amorphous materials - Materials, including glasses, that have no long-range order, or crystal structure. But amorphous does not mean random, in many cases there is some form of short-range order amorphous SiO2 structure