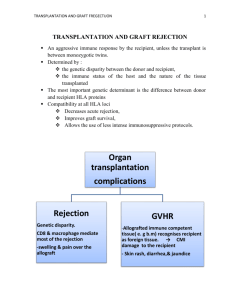
TRANSPLANTATION IMMUNOLOGY. Hilu Rashad, MD Student. Introduction ⚫ The field of organ transplantation has made remarkable progress in a short period of time. ⚫ Transplantation has evolved to become the treatment of choice for end-stage organ failure resulting from almost any of a wide variety of causes. ⚫ Transplantation of the skin, kidney, liver, pancreas, intestine, heart, and lungs has now become common in all parts of the world. Definition of Transplantation ⚫ Implantation of “non-self” tissue into the body ⚫ The process of taking cells, tissues, or organs called a graft (transplant), from one part or individual and placing them into another (usually different individual). ⚫ donor: the individual who provides the graft. ⚫ recipient or host: the individual who receives the graft. Methods of Transplantation ⚫ Autologous graft (autograft) –from one site to another one in the same individual (e.g., the use of a patient’s own skin to cover third-degree burns or a saphenous vein femoropopliteal graft) ⚫ Isogenic (isograft) – between genetically identical individuals of the same species (e.g., kidney transplant between identical twins). ⚫ Allogeneic (allograft or homograft) -transplantation of tissue between genetically non identical members of the same species (e.g., cadaver , live donor solid organ transplant ) ⚫ Xenogeneic (xenograft) –transplantation of tissue between members of different species (e.g., baboon kidney into a human). Transplant Antigens ⚫ The main antigens involved in triggering rejection are coded for by a group of genes known as the major histocompatibility complex (MHC). ⚫ In humans, the MHC complex is known as the human leukocyte antigen (HLA) system. It comprises a series of genes located on short arm of chromosome 6. Major Histocompatibility Antigens ⚫ It includes 3 regions: class Ia (loci A, B, C) class Ib (loci E, F, G, H), class II (loci DR, DQ, DP) and class III ⚫ Histocompatibility antigens are cell surface expressed on all cells (class I) and on APC, B cells, monocytes/macrophages (class II) ⚫ They are targets for rejection ⚫ They are inherited from both parents as MHC haplotypes and are co-dominantly expressed ⚫ HLA molecules can initiate rejection damage, via humoral or cellular mechanisms: and graft Humoral rejection mediated by recepient's antibodies. (e.g. blood transfusion, previous transplant, or pregnancy) Cellular rejection is the more common type of rejection after organ transplants. Mediated by T lymphocytes, it results from their activation and proliferation after exposure to donor MHC MECHANISMS OF REJECTION Rules of Transplantation Recognition of Alloantigens ⚫ Direct Presentation A MHC molecule is displayed by antigen-presenting cells (APCs) in the graft and recognized by recipient T cells without a need for host APCs. ⚫ Indirect Presentation Donor MHC molecules are captured and processed by recipient APCs and then presented to T cells Direct and Indirect Recognition Figure 16-3 Difference between Direct Recognition and Indirect Recognition 23 Effector Functions of Alloreactive T Cells ⚫ Alloreactive CD4+ and CD8+ T cells that are activated by graft alloantigens cause rejection by distinct mechanisms. ⚫ The CD4+ helper T cells differentiate into cytokine producing effector cells that damage grafts by cytokine mediated inflammation, similar to a delayed-type hypersensitivity (DTH) reaction ⚫ Alloreactive CD8+ T cells differentiate into cytotoxic T lymphocytes (CTLs), which kill nucleated cells in the graft that express the allogeneic class I MHC molecules. ⚫ CTLs also secrete inflammatory cytokines, which can contribute to graft damage. Activation of Alloreactive B Cells and Production of Alloantibodies ⚫ Most high-affinity alloantibodies are produced by helper T cell– dependent activation of alloreactive B cells, much like antibodies against other protein antigens ⚫ The antigens most frequently recognized by alloantibodies in graft rejection are donor HLA molecules,including both class I and class II MHC proteins. ⚫ The naive B lymphocytes recognize foreign MHC molecules, internalize and process these proteins, and present peptides derived from them to helper T cells Thus, producing Alloantibodies which cause allograft rejection. TYPES OF REJECTION ⚫ Hyperacute rejection is characterized by thrombotic occlusion of the graft vasculature that begins within minutes to hours after host blood vessels are anastomosed to graft vessels and is mediated by preexisting antibodies in the host circulation that bind to donor endothelial antigens ⚫ Acute rejection is a process of injury to the graft parenchyma and blood vessels mediated by alloreactive T cells and antibodies . TYPES OF REJECTION ⚫ Chronic rejection various mechanisms: cell-mediated, deposition of antibodies or antigen antibody complexes with subsequent obliteration of blood vessels and interstitial fibrosis ⚫ A dominant lesion of chronic rejection in vascularized grafts is arterial occlusion as a result of the proliferation of intimal smooth muscle cells, and the grafts fail because of the ischemic damage. Rate of rejection: The rate of rejection depends on the type underlying effector mechanisms: Type of rejection Time taken cause Hyperacute Min-hours Anti-donor Ab and complement Accelerated Days Reactivation of T cells Acute Days- weeks Primary activation of T cells Chronic Months- Years Unclear GRAFT VERSUS HOST DISEASE (GVH) ⚫ Graft mounts an immune response against the antigens of host . ⚫ Is common complication in recipients of bone marrow transplants ⚫ Is due to the presence of alloreactive T cells in the graft ⚫ It results in severe tissue damage, particularly to the skin and intestine ⚫ It may be avoided by careful typing, removal of mature T cells from the graft and by immunosuppressive drugs ⚫ It is manifested by marked rise of several cytokines in patient’s serum (IFN-, TNF, IL-1, IL-2, IL-4) RISK FACTORS IN FORMATION OF GVH Acute GVH Chronic GVH ⚫ Previous pregnancies in ⚫ Aging of donor and recipient female donor ⚫ High T cell number in marrow ⚫ HLA disparity ⚫ Transplant from female to male ⚫ Low immunosuppression ⚫ Herpes virus infection ⚫ Donor’s leukocyte transfusion ⚫ Previous acute GVH ⚫ High dosage radiation ⚫ Transplant from female to man ⚫ HLA disparity Prevention of transplant rejection ⚫ Tissue Typing ABO and Rh blood typing Cross matching (Preformed antibodies) HLA typing ⚫ Immunosuppressive Therapy ⚫ The pretransplant laboratory evaluation & immunization ⚫ Pre/post transplant prophylaxis Most Common Transplantation -Blood Transfusion- Transfuse Not transfused Methods of HLA typing ⚫ Microcytotoxicity test ⚫ Molecular methods -RFLP/PCR ⚫ Tissue matching- Mixed lymphocyte reaction( MLR) Mixed lymphocyte reaction (MLR). ⚫ The response of alloreactive T cells to foreign MHC molecules can be analyzed in an in vitro reaction called the mixed lymphocyte reaction (MLR). ⚫ MLR is a predictive test of T cell–mediated graft rejection. Pretransplant Evaluation ⚫ The pretransplant laboratory evaluation Tests to obtain in all transplant candidates Serologies Other tests Cytomegalovirus Urinalysis Herpes simplex virus Urine culture Varicella-zoster virus Tuberculin skin test or Epstein Barr virus Chest radiograph Human immunodeficiency virus Sputum stains and cultures Hepatitis B virus: HBsAg, HBsAb, HBcAb Hepatitis C virus Treponema pallidum Toxoplasma gondii (in heart transplant candidates) For bacteria, mycobacteria, and fungi (in lung transplant candidates) IMMUNISATION ⚫ Hepatitis B : Routine vaccine schedule recommended prior to transplant ⚫ If no tetanus booster in the past 10 years, then it should be administered. ⚫ Pneumovax should be administered before transplantation and repeated once 3-5 years after initial vaccination. • N. Meningitis vaccine: Recommended for patients Members of the military Travellers to high risk areas Properdin deficient Terminal complement component deficient Those with functional or anatomic asplenia • Rabies: Not routinely administered. Recommended for exposures or potential exposure • Varicella Zoster Vaccine : indicated for persons ≥ 60 years • Patients listed for organ transplantation should undergo tuberculin skin testing. • Baseline chest radiographs should be obtained for anyone with epidemiologic history suggestive of possible exposure. • Pretransplant anti-tuberculous prophylaxis or therapy for following specific indications: Tuberculin reactivity of ≥ 5 mm before transplantation History of tuberculin reactivity without adequate prophylaxis Recent conversion of tuberculin skin test to positive Radiographic evidence of old TB. A chest CT scan is performed to look for disseminated disease and to serve as a baseline study. History of inadequately treated TB Close contact with an individual with active pulmonary TB Receipt of an allograft from a donor with a history of untreated TB Common Types of Infecting Microbial Agents after Transplantation Bacteria Viruses Gram-negative bacteria Enteric bacteria (Escherichia coli, other Enterobacteriaceae) Pseudomonas Acinetobacter Serratia Bacteroides and other anaerobes Legionella Gram-positive aerobes Staphylococcus aureus (MRSA) Staphylococcus epidermidis Streptococcus Enterococcus (VRE) Pneumococcus Listeria monocytogenes Nocardia Gram-negative coccobacilli Haemophilus influenzae Herpes simplex virus (HSV) Cytomegalovirus (CMV) Varicella-zoster virus Epstein-Barr virus (EBV) Human herpesvirus-6 Human herpesvirus-8 Human immunodeficiency virus 1 (HIV1) Adenovirus Rotavirus Respiratory syncytial virus Influenza A and B viruses Para influenza viruses West Nile virus Hepatitis B virus Hepatitis C virus Polyomavirus Papillomavirus Parvovirus Common Types of Infecting Microbial Agents after Transplantation Fungi Mycoplasmas Candida spp. Aspergillus spp. Cryptococcus Agents of mucormycoses Histoplasma capsulatum Coccidioides immitis Pneumocystis jirovecii Mycoplasma hominis Ehrlichia Ehrlichia chafeensis Anaplasma phagocytophilum Protozoa and Parasites Toxoplasma gondii Trypanosoma cruzi Strongyloides stercoralis Timetable of infection after solid (renal) transplantation. HSV, CMV, EBV, VZV, Papova, TB. Prevention of infection post-transplant Marty and RubinTransplant International 19 (2006) 2–11 Antimicrobial Prophylactic Regimens in Transplantation Pathogen Protozoa Toxoplasmosis ,Pyrimethamine Viral Herpes simplex Cytomegalovirus ,Foscarnet Influenza Fungal Candida Aspergillus Pneumocystis Bacterial Wound infection Urinary tract infection Neutropenic infection Tuberculosis Prophylactic Agents Trimethoprim-sulfamethoxazole Acyclovir Ganciclovir, Acyclovir ,Immunoglobulin Amantadine, Rimantadine, Oesiltamivir Fluconazole, Nystatin, Clotrimazole Voriconazole Amphotericin B, Liposomal amphotericin TMP-SMX, Dapsone,Inhaled pentamadine Variable TMP-SMX Quinolones Isoniazid Immunosuppressive Therapy • The calcineurin inhibitors cyclosporine and FK506 (tacrolimus) inhibit transcription of certain genes in T cells, most notably those encoding cytokines such as IL-2. • Rapamycin (sirolimus) inhibits growth factor–mediated T cell proliferation • Antimetabolites are metabolic toxins that kill proliferating T cells eg. mycophenolate mofetil(MMF). • Azathioprine, Cyclophosphamide • Block the proliferation of lymphocytes • Anti-inflammatory agents • Corticosteroids----Block the synthesis and secretion of cytokines PERSPECTIVES OF XENOGENEIC GRAFTS • Potential advantage due to larger accessibility of animal organs • Monkeys are apparently the most suitable donors, but dangerous because of potential risk of retrovirus transfer within graft • Pigs are now considered because of similar sizes of organs and erythrocytes to human ones • The major obstacle – presence in man (1%) of natural antibodies vs. Gal (galactose--1,3-galactose) causing hyperacute rejection SUMMARY • Major histocompatibility complex is the main antigens involved in triggering transplant rejection . • HLA molecules initiates rejection and graft damage, via humoral or cellular mechanisms. • Donor alloantigens are presented by APCs to T lymphocytes of recipient by direct/ indirect methods SUMMARY • Immature dendritic cells within the graft carry donor antigens from the transplanted organ to the recipient’s draining lymph nodes ; during their journey, these antigens mature into APCs • The APCs then home to lymphoid organs • Here they activate the recipient’s T cells. • These T cells differentiate into various subgroups and return to the graft and destroy the transplanted organ SUMMARY • Rejection can be acute , hyperacute and chronic. • GVHD - graft mounts an immune response against the antigens of host . • Transplant rejection can be prevented by tissue typing & proper screening of the patients for infections. • Every transplant patient should get appropriate immunization & prophylactic drugs.