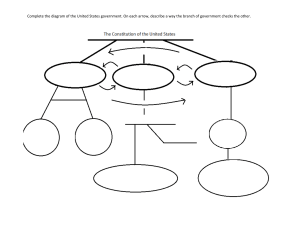
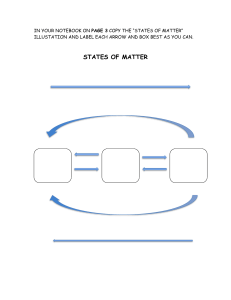
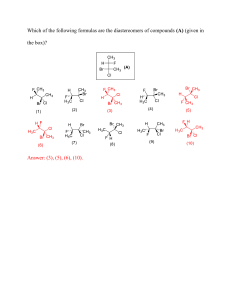
II. Chemical Reactions A. Chemical reactions describe a chemical change in which the atoms of compounds are rearranged to form new compounds. B. Chemical reactions are described with a chemical equation. The general format for a chemical equation is: A + B ------> C + D An example you are familiar with is the photosynthesis reaction: CO2 + H2O ------> C6H12O6 + O2 C. The compounds on the left hand side of the arrow are called the reactants. D. The compounds on the right hand side of the arrow are called the products. E. When you read the equation out loud, you read the arrow as “yields” or “produces”. F. Because the equation represents a chemical change, the properties of the reactants are different from the properties of the products.