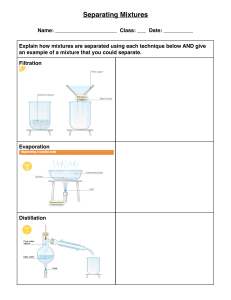
Which is a characteristic of mixtures? Answer: Mixtures are a combination of two or more substances that are physically combined, but not chemically bonded. A characteristic of mixtures is that they can be separated using physical methods such as filtration, distillation, or chromatography. Unlike compounds, which are formed by chemical bonding and cannot be separated by physical methods, mixtures retain the properties of their individual components. Mixtures can be heterogeneous, meaning that they have non-uniform compositions throughout the sample, or homogeneous, meaning that they have the same composition throughout the sample. In addition, mixtures can have different physical properties depending on their composition. For example, a mixture of salt and water may have different properties than a mixture of sugar and water. These physical properties can include color, density, boiling point, freezing point, and solubility. Furthermore, mixtures can be classified into different types depending on their composition, such as solutions, suspensions, colloids, and emulsions. For instance, a solution is a type of mixture that contains a solute uniformly dissolved in a solvent, while a colloid is a mixture in which small particles of one substance are dispersed throughout another substance. In conclusion, a characteristic of mixtures is that they can be separated using physical methods, they retain the properties of their individual components and can have different physical properties depending on their composition. Understanding the properties and types of mixtures is important in many fields, including chemistry, biology, and environmental sciences. References: - Chang, R. (2010). Chemistry (10th ed.). McGraw Hill. - McMurry, J., & Fay, R. C. (2015). Chemistry (7th ed.). Pearson. - Tro, N. J. (2017). Chemistry: A molecular approach (4th ed.). Pearson.