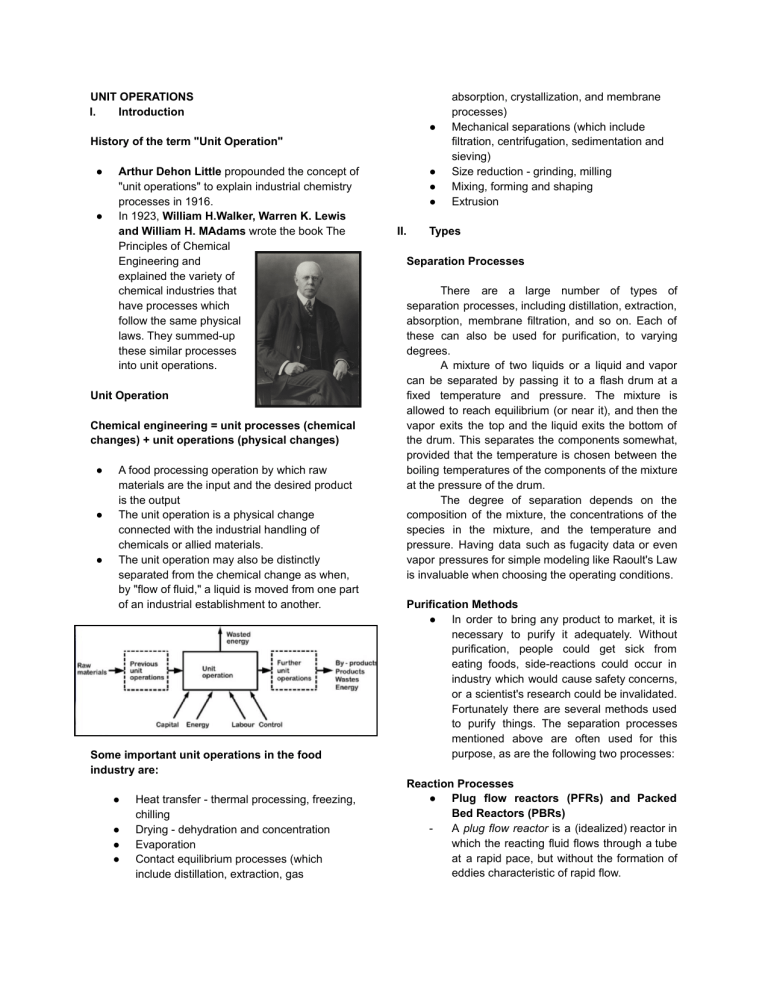
UNIT OPERATIONS I. Introduction ● History of the term "Unit Operation" ● ● Arthur Dehon Little propounded the concept of "unit operations" to explain industrial chemistry processes in 1916. In 1923, William H.Walker, Warren K. Lewis and William H. MAdams wrote the book The Principles of Chemical Engineering and explained the variety of chemical industries that have processes which follow the same physical laws. They summed-up these similar processes into unit operations. Unit Operation Chemical engineering = unit processes (chemical changes) + unit operations (physical changes) ● ● ● A food processing operation by which raw materials are the input and the desired product is the output The unit operation is a physical change connected with the industrial handling of chemicals or allied materials. The unit operation may also be distinctly separated from the chemical change as when, by "flow of fluid," a liquid is moved from one part of an industrial establishment to another. Some important unit operations in the food industry are: ● ● ● ● Heat transfer - thermal processing, freezing, chilling Drying - dehydration and concentration Evaporation Contact equilibrium processes (which include distillation, extraction, gas ● ● ● II. absorption, crystallization, and membrane processes) Mechanical separations (which include filtration, centrifugation, sedimentation and sieving) Size reduction - grinding, milling Mixing, forming and shaping Extrusion Types Separation Processes There are a large number of types of separation processes, including distillation, extraction, absorption, membrane filtration, and so on. Each of these can also be used for purification, to varying degrees. A mixture of two liquids or a liquid and vapor can be separated by passing it to a flash drum at a fixed temperature and pressure. The mixture is allowed to reach equilibrium (or near it), and then the vapor exits the top and the liquid exits the bottom of the drum. This separates the components somewhat, provided that the temperature is chosen between the boiling temperatures of the components of the mixture at the pressure of the drum. The degree of separation depends on the composition of the mixture, the concentrations of the species in the mixture, and the temperature and pressure. Having data such as fugacity data or even vapor pressures for simple modeling like Raoult's Law is invaluable when choosing the operating conditions. Purification Methods ● In order to bring any product to market, it is necessary to purify it adequately. Without purification, people could get sick from eating foods, side-reactions could occur in industry which would cause safety concerns, or a scientist's research could be invalidated. Fortunately there are several methods used to purify things. The separation processes mentioned above are often used for this purpose, as are the following two processes: Reaction Processes ● Plug flow reactors (PFRs) and Packed Bed Reactors (PBRs) A plug flow reactor is a (idealized) reactor in which the reacting fluid flows through a tube at a rapid pace, but without the formation of eddies characteristic of rapid flow. - - ● Plug flow reactors tend to be relatively easy to construct (they're essentially pipes) but are problematic in reactions which work better when reactants (or products!) are dilute. Plug flow reactors can be combined with membrane separators in order to increase the yield of a reactor. The products are selectively pulled out of the reactor as they are made so that the equilibrium in the reactor itself continues to shift towards making more products. Continuous Stirred-Tank Reactors (CSTRs) and Fluidized Bed Reactors (FBs) A continuous stirred-tank reactor is an idealized reactor in which the reactants are dumped in one large tank, allowed to react, and then the products (and unused reactants) are released out of the bottom. In this way the reactants are kept relatively dilute, so the temperatures in the reactor are generally lower. This also can have advantages or disadvantages for the selectivity of the reaction, depending on whether the desired reaction is faster or slower than the undesired one. Bioreactors ● A bioreactor is a reactor that utilizes either a living organism or one or more enzymes from a living organism to accomplish a certain chemical transformation. Bioreactors can be either CSTRs (in which case they are known as chemostats) or PFRs. Heat Exchangers ● A heat exchanger is a device which is used to facilitate the exchange of heat between two mixtures, from the hotter one to the cooler one. ● Heat exchangers very often involve steam because steam is very good at carrying heat by convection, and it also has a high heat capacity so it won't change temperature as much as another working fluid would. In addition, though steam can be expensive to produce, it is likely to be less expensive than other working fluids since it comes from water. III. Examples and Application in Chemical Industries/Process Distillation ● A liquid components are separated from the liquid mixture by partial vaporization by virtue of difference in vapor pressure ● Involves the separation of materials based on differences in their volatility ● Concentration of the more volatile component of the liquid mixture is higher in the vapor phase ● Concentration of less volatile component is higher in liquid phase Application: ● Separation of petroleum crude oil into LPG, gasoline,naphtha, kerosene, diesel, fuel oil, lubricating oil, and etc. ● Crude oil contains a variety of hydrocarbons that have different boiling points. ● The distilled products are then piped off from the different levels called fractions or distillates. Drying ● Separation of liquid moisture from the wet solids. ● Process where the liquid portion of the solution (moisture content) is evaporated ● Heat is supplied to vaporize the water ● Categorized into direct (convection), indirect (conduction), radiant (radiation) and dielectric or microwave (radio frequency) drying ● Last operation of any industry, Application: Drying in textile industry ● ● A textile dryer is used to remove moisture content from fabric or garments. This machine consists of two chambers. Two mesh endless conveyors are placed lengthwise named conveyor net and filter net which both contain a burner, which supplies hot air. ● There are nozzles placed in between filter net and conveyor net. When the fabric passes on the conveyor net, hot air is supplied to the wet fabric to dry it. Crystallization ● It is the process where solids particles are formed by the liquid solution. It comes under the solid liquid mass transfer operation. ● Crystallization can be done as a continuous or a batch process, depending on the capabilities of your crystallizer equipment. Typically, crystallizers are sized according to the volume of material that can be crystallized per hour. Two primary types of Crystallization ● Evaporative crystallization - the solution containing the solvent and the soluble component to be crystallized is heated until the solvent evaporates. ○ The evaporation of the solvent causes the molecules of the soluble compound to separate out as crystals due to the higher concentration exceeding the chemical compound’s solubility ● Cooling crystallization - when the solubility of the product increases significantly with increasing temperature. In a cooling crystallization process the feed is cooled in a heat exchanger, which can be situated inside the crystallizer or an external loop. Application ● Common application of crystallization in industry is its use to obtain pure salt from seawater. Salt crystallization is the most practical use of crystallization, and it is also the most cost-effective technique to create salt today. ● Crystallization can also be used to obtain pure alum crystals from an impure alum. In such scenarios, crystallization is known to be more effective than evaporation since it also removes the soluble impurities. Adsorption ● It is a unit operation that exploits the attraction of solutes in a liquid or gas to a solid surface. This attraction allows the solutes to be removed, or solutes with different affinities for the solid to be separated. ● Two primary types of Adsorption System: ○ Fixed-Bed - adsorbers utilize a stationary adsorbent to filter streams. ○ Fluidized-Bed - Adsorbers utilize a more complex moving (fluidized) adsorbent to filter streams. Application ● Purification of water by activated carbon or charcoal ● Removal of mixture from air by using the silica gel. Solvent Extraction ● A countercurrent separation method for separating the components of a liquid mixture is called solvent extraction. In its most straightforward form, this entails removing a solute from a binary solution by mixing it with a second immiscible solvent that the solute is soluble in. ● The most basic extraction procedure is single-contact batch extraction, which involves agitating the initial feed solution with a suitable solvent, allowing it to separate into two phases, and then decanting the solvent that contains the extracted solute. This is comparable to the lab technique using a separating funnel. Application ● Water Effluent Treatment ● Purification of Substances ● Food Industry: Cooking oil extraction from seeds Mixing ● A unit operation known as mixing entails the management of a physically heterogeneous system with the goal of increasing its homogeneity. ● Heat and/or mass transfer between one or more streams, components, or phases is made possible through mixing. Mixing is almost always a part of modern industrial operations. Application ● Improving product quality ● To coat particles ● Fusing materials Summary In chemical engineering and related fields, a unit operation is a basic step in a process. Unit operations involve a physical change or chemical transformation such as separation, crystallization, evaporation, filtration, polymerization, isomerization, and other reactions References BYJU'S. (2022, July 5). Crystallization definition, process, separation technique, faqs. BYJUS. Retrieved January 23, 2023, from https://byjus.com/chemistry/crystallization/#:~:t ext=Crystallization%20Examples&text=Co pper%20sulphate%20crystallises%20with%20 5,further%20uses%20for%20the%20technolog y. Dupre, C. (2020). What are the main impurities in crude oil? what are its common refining methods? Quora. Retrieved January 22, 2023, from https://www.quora.com/What-are-the-main-imp urities-in-crude-oil-What-are-its-common-refini ng-methods/answer/Charles-Dupre Joshi, M. (2021, April 25). Unit operations in Chemical Engineering with examples. Unit Operations in Chemical Engineering with examples. Retrieved January 22, 2023, from https://www.learnchems.com/2021/02/unit-oper ations-in-chemical-engineering-with-examples. html?fbclid=IwAR3Qhw6-XTk4X_vEw-uDAnC0 U4KXfCV0koclO-R_t3y9O0Y2wA7bKJg3gFw Kiron, M. I., & Mazharul Islam KironFounder & Editor of Textile Learner. He is a Textile Consultant. (2021, June 18). Drying machine in textile industry: Types, working principle & parameters. Textile Learner. Retrieved January 22, 2023, from https://textilelearner.net/drying-machine-in-texti le/#:~:text=Drying%20is%20defined%20as%20 a,which%20makes%20the%20water%20evapo rate. Tummolo, A. (2018, August 27). Evaporative vs. cooling crystallization systems. Thermal Kinetics. Retrieved January 23, 2023, from https://thermalkinetics.net/evaporative-vs-cooli ng-crystallization-systems/#:~:text=Evaporative %20Crystallization&text=The%20evapora tion%20of%20the%20solvent,exceeding%20th e%20chemical%20compound's%20solubility.