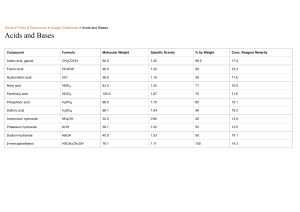
ACIDS, BASES AND SALTS Objectives At the end of the lesson, the students should be able to Define acids and bases Mention the physical properties of acids and bases Explain the chemical properties of acids of acids and bases Perform simple calculations on pH and pOH Define and classify salts Describe the types of salts Describe the methods of preparing salts Explain salt hydrolysis Key words Acid Base Salt Hydrolysis WEEK 1, PERIOD 1: Definition of acids and bases Acids and bases have been defined differently by different scientists. Svante Arrhenius, a Swedish scientist defined acids as substances that produce hydrogen ions as the only positive ions when dissolved in water and bases as substances that produce hydroxide ions as the only negative ions in water. Examples of Arrhenius’acid H2SO4, HCl, HNO3, H2CO3, HClO4 Examples of Arrhenius’ base NaOH, KOH, Ca(OH)2 Johannes Bronsted, a Danish scientist and Thomas Lowry, an American scientist had same views for the definition of acids and bases. They individually and at different locations defined acids as proton donors and bases as proton acceptors. Examples of Bronsted- Lowry acid All Arrhenius’ acids are Bronsted-Lowry acids. Other examples of Bronsted-Lowry acid include NH3, H2O(l) Examples of Bronsted- Lowry base NH3, H2O(l) Gilbert Newton Lewis, an American scientist defined acids as electron pair acceptors and bases as electron pair donors. Lewis’acids are characterized by having an orbital to accommodate electrons while Lewis’ bases are characterized by having lone pair of electrons. Examples of Lewis’ acid BF3, AlCl3, BCl3 Examples of Lewis’ base All Bronsted-Lowry bases are Lewis’s bases. Other examples of Lewis’ base include NH3, PH3, PCl3 Types of acids Acids are two types based on their source. These include inorganic or mineral acids and organic acids. Inorganic acids Inorganic acids are prepared from naturally occurring compounds called minerals such as nitrates, sulphates, chlorides. Hence they are referred to as mineral acids. Examples of inorganic acids Hydrochloric acid (HCl), trioxonitrate (V) acid (HNO3), tetraoxosulphate (VI) acid (H2SO4), trioxocarbonate (IV) acid (H2CO3), trioxosulphate (IV) acid (H2SO4). Organic acids Organic acids are derived from plants and animals. Hence they are naturally occurring acids. They are also known as alkanoic acids because they are derivatives of the hydrocarbons called alkanes. They can also be referred to as carboxylic acids because they possess the carboxyl group (-COOH) as functional group. Examples of organic acids Ethanoic acid (acetic acid), ethanedioic acid (oxalic acid), 2-hydroxypropanoic acid (lactic acid), hexadecanoic acid (palmitic acid), octadecanoic acid (stearic acid), 2hydroxypropane-1, 2, 3 – trioic acid (citric acid) Classification of acids Acids are classified based on concentration, strength and basicity. Based on concentration, acids are either concentrated or dilute. Concentrated acids contain a high proportion of the acid in a specific volume of water. Dilute acids contain a less proportion of the acid in a given volume of water. Based on strength, acids are either weak or strong. Weak acids ionize slightly in water and strong acids ionize completely in water. Examples of weak acids Hydrofluoric acid (HF), dioxonitrate (III) acid (HNO2), trioxocarbonate (IV) acid (H2CO3), trioxosulphate (IV) acid (H2SO3), trioxophosphate (III) acid (H3PO3), tetraoxophosphate (V) acid (H3PO4), all organic acids such as ethanoic acid (CH3COOH), ethanedioic acid (COOH)2 Examples of strong acids Hydrochloric acid (HCl), hydrobromic acid (HBr), hydroiodic acid HI), tetraoxochlorate (VII) acid (HClO4), trioxonitrate (V) acid (HNO3), tetraoxosulphate(VI) acid (H2SO4) Based on basicity, acids may be monobasic, dibasic or tribasic. The basicity of an acid is the number of replaceable hydrogen ions in a molecule of the acid. Monobasic acids possess one replaceable hydrogen ion in a molecule of the acid. Examples of monobasic acid All hydrohalic acids such as HF, HCl, HBr and HI. Other monobasic acids include HNO2, HNO3, HOCl, HOCl4, hexadecanoic acid acid CH3CH2(CH2)13COOH, octadecanoic acid CH3CH2(CH2)15COOH Dibasic acids possess two replaceable hydrogen ions in a molecule of the acid. Examples of dibasic acid Trioxosulphate (IV) acid (H2SO3), tetraoxosulphate (VI) acid (H2SO4), trioxocarbonate (IV) acid (H2CO3), ethanedioic acid. Tribasic acids possess three replaceable hydrogen ions in a molecule of the acid. Examples of tribasic acid Trioxophosphate (III) acid, tetraoxophosphate (V) acid, 2 – hydroxypropane - 1, 2, 3trioic acid. Physical properties of acids Physical state Inorganic acids are colourless liquids. Organic acids are either colourless liquids or white solids. Oxalic acid is a white crystalline solid. Corrosiveness Concentrated solutions of acids are corrosive. Solubility in water All inorganic acids and most organic acids are water – soluble. Taste Acids taste sour Ability to conduct electricity Acids conduct electricity in solution because of the ions produced when dissolved in water hence they are electrolytes pH The pH values of acids are within the range of 1 and 6. In other words, pH value of acids is less than 7. Colour change with indicators Acids turn blue litmus red and have no effect on red litmus Acids turn methyl orange solution pink or red Acids have no colour change with phenolphthalein. Acids remain colourless with phenolphthalein. Chemical properties of acids Reaction with bases (neutralization reaction) Acids react with bases to form salt and water Reaction with metallic oxides (neutralization reaction) Acids react with metallic oxides to form salt and water Reaction with reactive metals (displacement reaction) Acids react with metals more reactive than hydrogen to produce salt and hydrogen Reaction with carbonates (metallic trioxocarbonate (IV) compounds) or bicarbonates (metallic hydrogen trioxocarbonate (IV) compounds) Acids react with carbonates or bicarbonates to produce salt, water and carbon (IV) oxide gas. Reaction with sulphites (metallic trioxosulphate (IV) compounds) or bisulphites (metallic hydrogen trioxosulphate (IV) compounds) Acids react with sulphites or bisulphites to produce salt, water and sulphur (IV) oxide gas. Reaction with metallic sulphides Acids react with metallic sulphides to produce salt and hydrogen sulphide gas. Laboratory preparation of acids Acids are prepared in the laboratory by four methods: Direct combination (preparation of hydracids) Dissolution of acid anhydride in water Reaction of non-metals with strong oxidizing agent like concentrated HNO3 Reaction of salts of more volatile acids with less volatile acids Uses of acids Acids have quite useful applications Types of bases Bases are two types based on their solubility in water. These include soluble bases and insoluble bases. Soluble bases Soluble bases are soluble in water. They are also referred to as alkalis. All alkalis are bases but not all bases are alkalis. Examples of soluble bases Sodium hydroxide (NaOH), potassium hydroxide (KOH), aqueous ammonia (NH3). Insoluble bases Insoluble bases are insoluble in water. Examples of insoluble bases Magnesium hydroxide Mg(OH)2, aluminum hydroxide Al(OH)3, copper (II) oxide (CuO), copper (II) hydroxide, zinc hydroxide, magnesium oxide, iron (II) hydroxide, iron (III) hydroxide Calcium hydroxide Ca(OH)2 is slightly water soluble Classification of bases Bases are classified based on concentration, strength and acidity. Based on concentration, bases are either concentrated or dilute. Concentrated bases contain a high proportion of the base in a specific volume of water. Dilute bases contain a less proportion of the base in a specific volume of water. Based on strength, bases are either weak or strong. Weak bases ionize slightly in water and strong bases ionize completely in water. Examples of weak bases Magnesium hydroxide, aluminum hydroxide, aqueous ammonia Examples of strong bases Sodium hydroxide, potassium hydroxide Based on acidity, bases may be monobasic, dibasic or tribasic. The acidity of a base is the number of ionizable hydroxide ions in a molecule of the base. Monoacidic bases possess one ionizable hydroxide ion in a molecule of the base. Examples of monoacidic base NaOH, KOH Diacidic bases possess two ionizable hydroxide ions in a molecule of the base. Examples of diacidic base Mg(OH)2, Ca(OH)2, Fe(OH)2, Cu(OH)2, Zn(OH)2 Triacidic bases possess three ionizable hydroxide ions in a molecule of the base. Examples of triacidic base Al(OH3, Fe(OH)3 Physical properties of bases Physical state Bases may be solids such as sodium hydroxide, potassium hydroxide or liquids such as aqueous ammonia. Touch Bases have a soapy feel Corrosiveness Concentrated solutions of bases are corrosive. Solubility in water Some bases are water-soluble while some are water-insoluble Taste Bases taste bitter Ability to conduct electricity Bases conduct electricity in solution because they produce ions when dissolved in water, hence they are electrolytes. pH The pH values of bases are within the range of 8 and 14. In other words, pH value of bases is greater than 7. Colour change with indicators Bases turn red litmus blue and have no effect on blue litmus Bases turn methyl orange solution yellow or orange Bases turn colourless phenolphthalein pink or red Chemical properties of bases Reaction with acids (neutralization reaction) Bases react with acids to form salt and water Reaction with ammonium salt Alkalis except ammonia react with ammonium salts to produce salt, water and ammonia gas. Thermal decomposition reaction Bases with the exception of sodium hydroxide and potassium hydroxide decompose on heating to produce their respective oxides and water. Reaction with carbon (IV) oxide Bases react with carbon (IV) oxide to produce trioxocarbonate (IV) of the metal and water Reaction of alkalis with some salts Alkalis react with solution of some salts such as salts containing metals like aluminum, lead, zinc, iron or copper to form insoluble metal hydroxides which can be identified by their distinct colour. Amphoteric nature of some bases Some bases such the hydroxides of aluminum, zinc and lead have dual properties as they react with acids as well as bases to form salt and water. Laboratory preparation of bases Bases are prepared in the laboratory by a number of methods: Reaction of metals with oxygen Dissolution of certain metals more reactive than hydrogen in cold water or steam Thermal decomposition of certain trioxocarbonate (IV) compounds Thermal decomposition of certain trioxonitrate (V) compounds Uses of bases Bases have numerous applications pH and pOH Meaning of pH p in pH stands for potenz in German which means strength or power in English. H stands for hydrogen Definition of pH The pH of a solution is the logarithmic reciprocal of the hydroxonium ion concentration or the negative logarithm to base ten of the hydroxonium ion concentration. Mathematical representation of pH For simplicity, the hydrogen ion is used in place of the hydroxonium ion or Definition of pOH The pOH of a solution is the logarithmic reciprocal of the hydroxide ion concentration or the negative logarithm to base ten of the hydroxide ion concentration. Mathematical representation of pOH or Calculating the pH of pure water Consider the ionization of water: H2O(l) H+(aq) + OH-(aq) Equilibrium constant (K) expression for the ionization of water: Ionic product of water (kw) [H2O(aq)]K = K= is the ionic product of water It’s been determine that the ionic product of water (kw) at 25 oC (298 K) is 10-14 mol2dm-6 For pure water, [H+} = 10-7 mol dm-3 and [OH-] = 10-7 moldm-3 Calculating pH of pure water pH = - Log1010-7 = -7(-Log1010) = -7 (-1) pH = 7 Calculating pOH of pure water pOH = - Log1010-7 = -7(-Log1010) = -7 (-1) pOH = 7 Writing the pkw of pure water The sum of the pH and pOH of pure water is called pkw and its value is 14 pkw = pH + pOH pH + pOH = 14 pH scale pH scale ranges from 1 to 14. pH values between 1 and 6 are acidic. pH value of 7 is neutral and pH values between 8 and 14, basic. Acidity increases with increase in hydroxonium ion concentration but decrease in the hydroxide ion concentration Therefore acidity increases with decrease in the value of pH. Basicity increases with increase in hydroxide ion concentration but decrease in the hydroxonium ion concentration Therefore basicity increases with increase in the value of pH. The figure below shows the summary Measurement of pH pH can be measured by any of three methods: The use of litmus The use of universal indicator The use of pH meter Quiz 1. 2. 3. 4. 5. 6. 7. What is the pH of a 0.01 mol dm-3 solution of hydrochloric acid? What is the pOH of a 0.1 mol dm-3 solution of potassium hydroxide? What is the pH of a 0.0001 mol dm-3 solution of tetraoxosulphate (VI) acid? What is the pH of a 0.001 mol dm-3 solution of sodium hydroxide? What is the pOH of a 0.01 mol dm-3 solution of hydrochloric acid? What is the hydrogen ion concentration of a solution of pH 4.398? A sample of orange juice is found to have a pH of 3.80. What is the hydroxide ion concentration? 8. What is the pH of a 2.50 × 10-5 mol dm-3 solution of sodium hydroxide? 9. What is the pOH of a solution of 0.25 mol dm-3 of hydrochloric acid? 10. What is the pOH of a 0.05 mol dm-3 solution of calcium hydroxide? Definition of salts A salt is a compound formed by the replacement of the hydrogen ion in an acid with a metallic ion or ammonium radical. Types of salts Salts are of six types and they include Normal salts A normal salt is one formed by the complete replacement of ionizable hydrogen ion of an acid by a metallic ion. Hence does not contain any replaceable hydrogen ion in its molecule. Most are neutral to litmus such as NaCl(aq), KCl(aq),Na2SO4(aq), KNO3(aq), but some are acidic or basic. For instance, NH4Cl(aq) is slightly acidic and CH3COONa(aq) is slightly basic. Acid salts An acid salt is one formed by the partial replacement of the ionizable hydrogen ion in an acid with metallic ions. Hence it contains replaceable hydrogen ion in its molecule. NaHSO4(aq) and KHSO4(aq)are acidic in solution because they formed by the reaction between a strong acid and a strong base and contain ionizable hydrogen ion but some acid salts such as NaHCO3(aq), KHCO3(aq), Na2HPO4(aq) are slightly alkaline in solution because they are formed by the reaction between a weak acid and a strong base. Acid salts ionize in solution to form hydroxonium ion and react with bases to form normal salts. Examples of acid salt NaHSO4(aq), KHSO4(aq), NaHCO3(aq), KHCO3(aq), NaH2PO4(aq), Na2HPO4(aq) Basic salts A basic salt is one formed by the partial replacement of the hydroxide ion in a base with an anion. Basic salts are not necessarily alkaline to litmus and reacts with acid to form normal salt Examples of basic salts Zn(OH)Cl, Pb(OH)Cl, Pb(OH)NO3, Cu(OH)NO3, Cu(OH)Cl Double salts A double salt is formed when stoichiometric (calculated) amounts of two simple salts combine and exists in the solid state but dissociate into their constituent simple ions when dissolved in water. Examples of double salts Potash alum K2SO4.Al2(SO4)3.24H2O, Mohr’s salt FeSO4(NH4)2SO4.6H2O, carnalite KCl.MgCl2.6H2O Complex salts A complex salt is a compound in which the central metal ion is bonded to a number of neutral molecules, anions or cations by coordinate bonds. The coordinate bonds are formed by the donation of electron by neutral molecule, anions or cations to the metal ion. Examples of complex salts [Cu(NH3)4](OH)2, K3[Fe(CN)6], K3[Fe(CN)6] K+ + Simple ion [Fe(CN)6]3complex ion Mixed salts A mixed salt is one that contains more than one acidic or basic radical other than hydrogen or hydroxide ions. Examples of mixed salts CaOCl2 (bleaching powder), KNaCO3, CaKPO4, NH4NaHPO4 Classification of salts Salts are classified based on their solubility in water into soluble salts and insoluble salts, as well as based on their stability to heat. Soluble salts are water soluble. Examples of soluble salts All sodium containing salts All potassium containing salts All ammonium salts All dioxonitrate (III) salts All trioxonitrate (V) salts All bicarbonate (metallic hydrogen trioxocarbonate (IV) salts All ethanoate salts All tetraoxosulphate (VI) salts except those containing calcium, barium, mercury (I), lead or silver All chlorides except those containing silver, lead or mercury (I) Examples of insoluble salts All sulphides All carbonates except those containing sodium, potassium or the ammonium radical All tetraoxophosphate (V) salts except those containing sodium, potassium or the ammonium radical All trioxophosphate (III) salts except those containing sodium, potassium or the ammonium radical All trioxosulphate (IV) salts except those containing sodium, potassium or the ammonium radical Based on stability to heat, salts are either stable to heat or unstable to heat. Salts stable to heat do not decompose on heating Examples of salts stable to heat Na2CO3, K2CO3 Salts unstable to heat decompose on heating Examples of salts unstable to heat CaCO3, NaHCO3, all trioxonitrate (V) salts Physical properties of salts Physical state Salts are solids at room temperature Colour Some salts appear white in colour. Examples include salts of sodium and potassium. Some other salts have characteristic colour such as blue (copper containing salt), green (iron (II) containing salt), brown (iron (III) containing salt), orange (dichromate containing salt). Solubility in water Some salts are water soluble while others are water insoluble. Some are said to be slightly or sparingly water soluble. Ability to conduct electricity Salts conduct electricity in the molten state or in solution because of the presence of ions when in these states. Hence they are electrolytes. Melting temperature The melting point of salt is quite high due to electrostatic force of attraction between the ions that make up the salt. Laboratory preparation of salts Salts are prepared in the laboratory according to their solubility in water. Methods of preparing water soluble salts Reaction of acids with bases Reaction of acids with metallic oxides Reaction of acids with reactive metals Reaction of acids with carbonates and bicarbonates Methods of preparing water insoluble salts Direct combination of the constituent elements of the salt Double decomposition reaction (precipitation reaction) Definition of salt hydrolysis Salt hydrolysis is the partial reversal of neutralization reaction when the metallic ions or acid radicals of the salt react with water to form a weak base or weak acid. Mechanism of salt hydrolysis Salts formed by the reaction between strong acids and strong bases do not undergo hydrolysis and their solution is neutral. This is because the acid and base that form them ionize completely in water. Examples of such salts include NaCl, K2SO4 Salts formed by the reaction between strong acids and weak bases undergo hydrolysis to produce an acidic solution. This is because the cation of the salt undergoes hydrolysis by reacting with the hydroxide ion from water to form solution of weak base with the formation of hydrogen ions. The hydrogen ions formed is responsible for the acidity of the salt solution. This is known as cation hydrolysis. Examples of such salts include NH4Cl, CaSO4, FeCl3. Consider the hydrolysis of NH4Cl(aq), In solution: NH4Cl(aq) H2O(l) NH4+(aq) + Cl-(aq) H+(aq) + OH-(aq) The cation from the salt combines with the hydroxide ion from water: NH4+(aq) + OH-(aq) NH3(aq) + H2O(l) (i) H2O(l) ionizes: H2O(l) H+(aq) + OH-(aq) (ii) Combining both ionic equations (i) and (ii) NH4+(aq) + OH-(aq) + H2O(l) NH3(aq) NH4+(aq) NH3(aq) + + H2O(l) + H+(aq) + OH-(aq) H+(aq) Responsible for acidity Salts formed by the reaction between weak acids and strong bases undergo hydrolysis to produce a basic solution.. This is because the anion of the salt undergoes hydrolysis by reacting with the hydrogen ion from water to form a solution of weak acid with the formation of hydroxide ions. The hydroxide ions formed is responsible for the basicity of the salt solution. This is known as anion hydrolysis. Examples of such salts include Na2CO3, KCN, Na2S, CH3COONa. Consider the hydrolysis of CH3COONa(aq), In solution: CH3COOH(aq) H2O(l) CH3COO-(aq) + H+(aq) H+(aq) + OH-(aq) The anion from the salt combines with the hydrogen ion from water: CH3COO-(aq) + H+(aq) CH3COOH(aq) (iii) H2O(l) ionizes: H2O(l) H+(aq) + OH-(aq) (iv) Combining both ionic equations (iii) and (iv) CH3COO-(aq) + H+(aq) + H2O(l) CH3COO-aq) + H2O(l) CH3COOH(aq) + H+(aq) + OH-(aq) CH3COOH(aq) + OH-(aq) Responsible for basicity Salts formed by the reaction between weak acids and weak bases undergo hydrolysis but whether the resulting salt solution is acidic or basic depends on the relative strength of the acid and base that formed the salt. Examples of such salts include CH3COONH4, (NH4)3PO4 . Multiple choice Quiz 1. Which of the following statements is not a chemical property of an acid? A. evolution of ammonia gas when heated with ammonium salts B. evolution of CO2 gas when added to a trioxocarbonate (IV) salts C. formation of salt and water with alkalis D. formation of salt and hydrogen gas with reactive metals 2. A substance L reacts with NH4NO3(aq) to generate ammonia gas. L is likely to be A. HCl C. CH3COOH B. NaOH D. CaSO4 3. Which of the following chlorides is insolubke in water? A. AgCl C NH4Cl B. KCl D. ZnCl2 4. Which of the fooling hydrohalic acids is the weakest? A. HBr C. HF B. HCl D. HI 5. Which of the following pH values indicates that a solution is a strong base? A. 1 C. 9 B. 5 D. 13 6. The hydrolysis of NH4Cl gives A. an acidic solution C. a buffer solution B. an alkaline solution D. a neutral solution 7. Which of the following acids would readily react with CaCO3 to liberate CO2? A. CH3COOH C. H2SO3 B. H2SO4 D. HNO3 8. Which of the following oxides is basic? A. NO2 C. SO2 B. Al2O3 D. CaO 9. An example of an acid salt is A. CH3COONa C. NaHSO4 B. Mg(OH)Cl D. (NH4)2SO4 10. The aqueous solution which has pH > 7 is A. FeCl3(aq) C. KNO3(aq) B. CuSO4(aq) D. Na2CO3(aq)