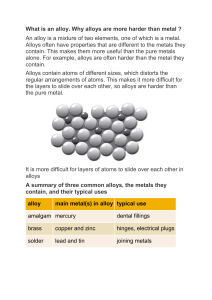
TOPIC: 2.4 STRUCTURE OF METALS AND ALLOYS ENDURING UNDERSTANDING: SAP-3 Atoms or ions bond due to interactions between them, forming molecules. LEARNING OBJECTIVE: SAP-3.D Represent a metallic solid and/or alloy using a model to show essential characteristics of the structure and interactions present in the substance. ESSENTIAL KNOWLEDGE: SAP-3.D.1 Metallic bonding can be represented as an array of positive metal ions surrounded by delocalized valence electrons (i.e., a “sea of electrons). SAP-3.D.2 Interstitial alloys form between atoms of different radii, where the smaller atoms fill the interstitial spaces between the larger atoms (e.g., with steel in which carbon occupies the interstices in iron). SAP-3.D.3 Substitutional alloys form between atoms of comparable radius, where one atom substitutes for the other in the lattice. (In certain brass alloys, other elements, usually zinc, substitute for copper.) EQUATION(S): N/A NOTES: Metals are composed of cations that are embedded in delocalized sea of valence electrons. This means that the electrons do not stay with one atom, rather they are able to move throughout the entire substance. The cations and the electrons are attracted to one another through a Coulombic Attraction which holds the metal atoms together. In this image you can’t tell which electron came from which cation, this is the idea of delocalization. https://www.assignmentpoint.com/science/chemistry/metallic-bond.html The number of valence electron determines the amount of electrons in the delocalized sea of electrons. When the charge on the cations and the number of electrons increases the attractions are greater. Additionally, when the ionic radius decreases the attraction increases. https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry__The_Central_Science_(Brown_et_al.)/11%3A_Liquids_and_Intermolecular_Forces/1 1.8%3A_Bonding_in_Solids Mixtures of metals are called alloys; they can be examples of a solution. Two types of alloys are interstitial and substitutional. The difference between them is the size of the atoms that are being added to the metal. Interstitial Substitutional The atoms added to the metal are small and fit in The atoms added to the metal have similar radii so between the metal atoms in the existing holes they replace the atoms in the lattice. (interstices). (H, B, C and N are commonly added) https://www.e-education.psu.edu/matse81/node/2141 I DO: Carbon steel is an alloy composed of a small amount of carbon atoms combined with iron. Consider the atomic radii of both carbon and iron and draw a model that describes the alloy that forms. Cafe key WE DO: c C fe 0000 intersh carbinia'nEs n smaller radius than iron m Examine the diagrams below, and then label each with the type of substance shown. https://chem.libretexts.org/Courses/University_of_Missouri/MU%3A__1330H_(Keller)/23%3A_ Metals_and_Metallurgy/23.6%3A_Alloys YOU DO: 1) Below are two examples of alloys, one is an alloy of tungsten with carbon while the other is copper and tin. Label the two types of alloy and create a key to label each type of atom. https://wps.prenhall.com/wps/media/objects/3313/3392987/blb2306.html 2) In 2018, scientists engineered an alloy composed of platinum and gold, which is 100 times more wearresistant than stainless steel. It is 90% platinum and 10% gold. Sketch a model of 20 atoms of this alloy, indicating if it is interstitial or substitutional, use the correct ratio of atoms and be sure to include a key. 3) Compare the metals calcium and magnesium, which metal would be stronger (more tightly held together), justify your selection. 4) A pure metal and an interstitial alloy containing the same metal were examined. Predict which substance will have a greater density and justify your selection. 5) Interstitial alloys are usually less malleable than the pure metals. Based on their structure, propose a reason for this decrease in malleability.