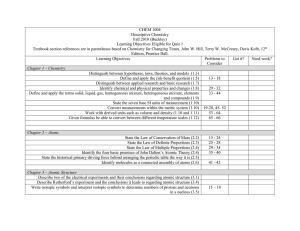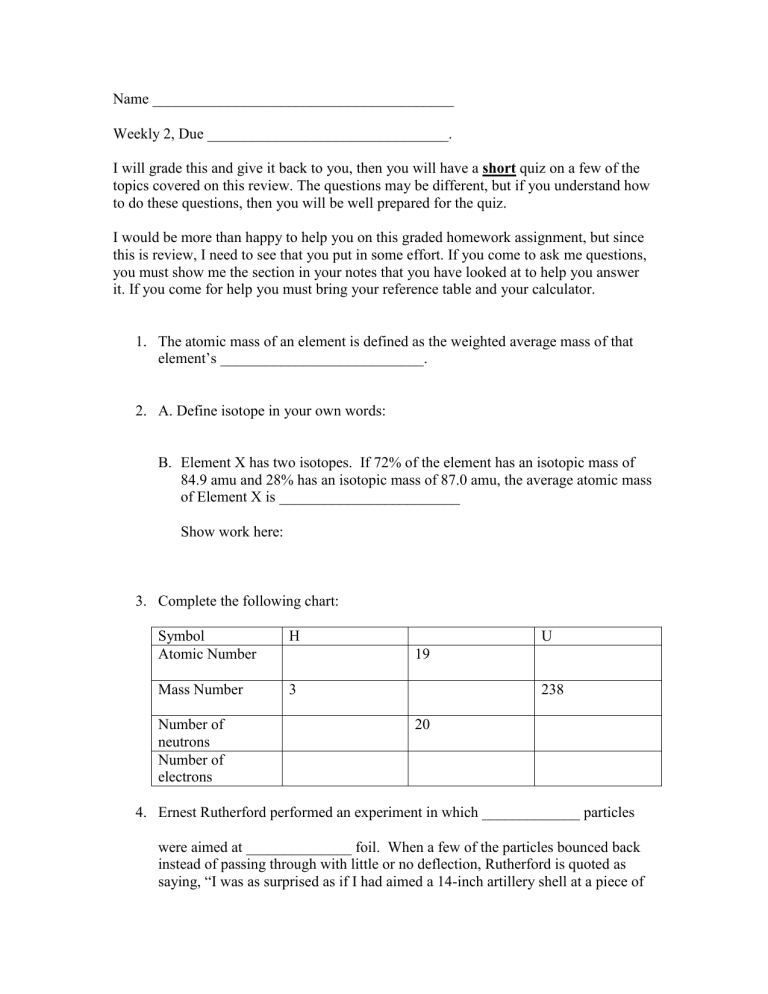
Name ________________________________________ Weekly 2, Due ________________________________. I will grade this and give it back to you, then you will have a short quiz on a few of the topics covered on this review. The questions may be different, but if you understand how to do these questions, then you will be well prepared for the quiz. I would be more than happy to help you on this graded homework assignment, but since this is review, I need to see that you put in some effort. If you come to ask me questions, you must show me the section in your notes that you have looked at to help you answer it. If you come for help you must bring your reference table and your calculator. 1. The atomic mass of an element is defined as the weighted average mass of that element’s ___________________________. 2. A. Define isotope in your own words: B. Element X has two isotopes. If 72% of the element has an isotopic mass of 84.9 amu and 28% has an isotopic mass of 87.0 amu, the average atomic mass of Element X is ________________________ Show work here: 3. Complete the following chart: Symbol Atomic Number H Mass Number 3 Number of neutrons Number of electrons U 19 238 20 4. Ernest Rutherford performed an experiment in which _____________ particles were aimed at ______________ foil. When a few of the particles bounced back instead of passing through with little or no deflection, Rutherford is quoted as saying, “I was as surprised as if I had aimed a 14-inch artillery shell at a piece of paper and it bounced back.” Why was he so surprised, and how did he modify the model of the atom to explain his result? 5. Draw the atomic model of the following: N Na Ca 6. Record this measurement to the correct number of sig figs. ________ cm 1 2 3 4 5 6 7 8 9 10 11 12 7. How many significant figures are in each of the following numbers? a. 203 b. 12,700 c. 7,720. d. 0.003 e. 0.460 8. Complete the following mathematical operations and round to the correct number of significant figures. a. 12.0 x 3.0 b. 23.1 + 6.58 c.0.40 + 1.32 d.6.5 ÷ 2.512 9. Complete the following table regarding the measurements: Measurement (cm) List known digits, if any List uncertain digits, if any # of significant figures 29.7 0.02310 160 10. Perform the following calculations, round your answer to the correct number of significant figures: 7.20 x 10-5 / 1.21 x 10-2 = ________________________________________ 4.68 x 1014 * 8.9031 x 1010 = _____________________________________