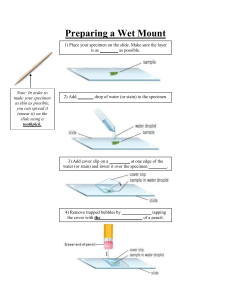
Activities I have observed during my placement at Serology LAB ( Virology Dept.) of IEDCR 1. Sample collection and preservation a. NISB Sample Nasal swab i. ILI( Influenza Like Illness) ii. SARI (Sever Acute Respiratory Illness) b. QC Sample: For cross checking purpose, DG Health Covid samples and LAB Protocol maintain c. SARS COV-02 Sample: Nasal swab sample d. ROTA Suspected Sample: Faecal specimen samples are collected and preserved at -80 degree for further tests 2. Observed Tests Dengu NS1 Rapid Test The Dengue NS1 Ag-IgM/gG Combo Test is a rapid chromatographic immunoassay for the qualitative detection of antibodies (IgM and IgG) and dengue virus NS1 antigen to dengue virus in Whole Blood /Serum / Plasma to aid in the diagnosis of Dengue viral infection. Kit: Dengue NS1 Ag-IgM/lgG Combo Test Sample: Blood Sample ( collected within three days of infection) Principle: The Dengue NS1 Ag-IgM/IgG Combo Test is a qualitative membrane strip based immunoassay for the detection of dengue virus antibodies (IgM and IgG) and dengue virus NS1 antigen in Whole Blood or Serum Plasma. For IgM/IgG Test: The test device consists of: 1) a burgundy colored conjugate pad containing dengue recombination envelope antigens conjugated with Colloid gold (dengue conjugates), 2) a nitrocellulose membrane strip containing two test lines (T1 and T2 lines) and a control line (C line). The T1 line is pre-coated with the antibody for the detection of IgM anti-dengue, T2 line is coated with antibody for the detection of IgG anti-dengue. When an adequate volume of test specimen is dispensed into the sample well of the test cassette, the specimen migrates by capillary action across the cassette. IgG anti-dengue, if present in the specimen, will bind to the dengue conjugates. The immunocomplex is then captured by the reagent pre-coated on the T2 band, forming a burgundy colored T2 line, indicating a dengue lgG positive test result and suggesting a recent or repeat infection. IM anti-dengue if present in the specimen will bind to the dengue conjugates. The immunocomplex is then captured by the reagent coated on the T1 line, forming a burgundy colored T1 line, indicating a dengue IgM positive test result and suggesting a fresh infection. Absence of any T lines or T1 and T2 suggests a negative result. For NS1 Test: In this test procedure, anti-Dengue NS1 antibody is immobilized in the test line region of the cassette. After a Whole Blood /Serum / Plasma specimen is placed in the specimen well, it reacts with anti-Dengue NS1 antibody coated particles that have been applied to the specimen pad. This mixture migrates chromatographically along the length of the test strip and interacts with the immobilized anti-Dengue NS1 antibody. If the specimen contains dengue virus NS1 antigen, a colored line will appear in the test line region indicating a positive result. If the specimen does not contain dengue virus NS1 antigen, a colored line will not appear in this region indicating a negative result. To serve as a procedural control, a colored line will always appear at the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred. Procedure: Allow the test, specimen, buffer and controls to reach room temperature 15-30°C prior to testing. i. Bring the pouch to room temperature before opening it. Remove the test device from the sealed pouch and use it as soon as possible. Place the test device on a clean and level surface. ii. For igM-IgG Test: Hold the dropper vertically and transfer 1 drop of specimen (approximately 10 μl) to the specimen wells of the test device, then add 2 drops of buffer (approximately 70 μl) and start the timer. iii. For NS1Test: For serum or plasma specimen: Hold the dropper vertically and transfer 810 drops of serum or plasma (approximately 100 μl) to the specimen wells of the test device, then start the timer. For whole blood specimens: Hold the dropper vertically and transfer 3 drops of whole blood(approximately 35 micro liter) to the specimen well of the test device, then add 2 drops of buffer (approximately 70 μl) and start the timer. iv. Wait for the colored lines to appear. Read results at 15 minutes. Do not interpret the result after 20 minutes. Interpretation of results: Positive: For IgM/lg Test: Control line and at least one test line appear on the membrane. The appearance of T2 test line indicates the presence of dengue specific IgG antibodies. The appearance of T1 test line indicates the presence of dengue specific IgM antibodies. And if both T1 and T2 line appear, It indicates that the presence of both dengue specific IgM and IgG antibodies. The lower the antibody concentration is, the weaker the result line is. For NS1 Test: Two lines appear. One line should always appear in the control line region(C), and another one apparent colored line should appear in the test line region. Negative: One colored line appears in the control region(C). No apparent colored line appear in the test line region. Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. ROTA ELISA Rotaviruses are non-enveloped RNA viruses of icosahedral symmetry consisting of a spherical inner core and two outer capsid shells. At least seven serogroups (A-G) within the genus Rotavirus have been identified Human serotypes of Group A rotavirus are a major cause of gastroenteritis in young children throughout the world. Rotavirus gastroenteritis also occurs in older children and elderly populations. The virus is commonly associated with nosocomial infections in pediatric wards and neonatal nurseries. Outbreaks can result in prolonged hospitalisation and treatment of infected children Rotavirus gastroenteritis may be severe, even life threatening, in undernourished or immunocompromised infants. Kit: ProSpect Rotavirus Microplate Assay Sample: Faecal specimens collected 3-5 days after the onset of symptoms Principle: The ProSpecT Rotavirus test utilises a polyclonal antibody in a solid phase sandwich enzyme immunoassay to detect group specific antigen present in Group A rotaviruses. Break-apart microwells are coated with a rotavirus specific polyclonal antibody. Faecal suspension or control sample is added to the microwell and incubated simultaneously with a rotavirus specific polyclonal antibody conjugated to horseradish peroxidase. Rotavirus antigen present in the sample is captured between antibody on the solid phase and the enzyme conjugated antibody. After 60 minutes incubation at room temperature, the microwells are washed with working strength Wash Buffer to remove excess specimen and any unbound enzyme labelled antibody. A chromogen is added to the microwells and incubated for 10 minutes at room temperature. The presence of specifically bound enzyme labelled antibody in the microwells results in a colour change, which is stopped by the addition of acid. Colour intensity significantly above background levels is indicative of the presence of rotavirus antigen in the specimen or control. Procedure: 1. Open the foil pouch, remove the required number of microplate strips and place into a microplate strip holder tiger. Use one well for the Negative Control and one well for morosre the Positive Control. If using less than 8 wells break off. 2. Add 2 drops (or 100 μl) of each diluted specimen, Negative Control or Positive Control to the separate microwells. At least one Negative Control and one Positive Control should sibut be included in each batch of tests. 3. After addition of all specimens and controls, add 2 drops Ariw m (or 100 μl) of Conjugate to each microwell. 4. Cover the plate and incubate the microwells at 20-30°C for 60 minutes. 5. Shake out or aspirate the contents of the wells. Wash by completely filling each well with diluted Wash Buffer (~350-400Tub per well). Shake out or aspirate all fluid from wells after each wash. Wash a total of 5 times. After the last wash remove contents and strike plate on clean paper towels or aspirate. If using an automated washer, this should be programmed to complete 5 wash cycles. Washers must be correctly calibrated to ensure complete filling and emptying of microwells between each wash. After the final wash, the plate should be inverted and tapped on absorbent paper to remove the last traces of wash buffer. 6. Add 2 drops (or 100 μl) of Substrate to each microwell. 7. Cover the plate and incubate the microwells at 20-30°C S for 10 minutes. 8. Stop the Substrate reaction by adding 2 drops (or 100 μl) of Stop Solution to each microwell. Ensure thorough mixing of the microwells before reading the results. The coloured product is stable for up to 30 minutes after addition of Stop Solution. Interpretation of results: SPECTROPHOTOMETRIC DETERMINATION The microwells should be read photometrically within 30 minutes of addition of the Stop Solution. Mix the contents of the microwells and read the absorbance of each microwell using a spectrophotometer set at 450 m. Ensure the bottoms of the microwells are clean before reading. The reader should be blanked on airbefore the plate is scanned. The spectrophotometer allows for the use of reference wavelength (at 620 to 650m), dual wavelength reading Calculate the cut-off value by adding 0.200 absorbance units to the Negative Control value, or mean value when more than one Negative Control is included. Interpret the test results: Positive: clinical sample absorbance value > the cutoff value. Negative: clinical sample absorbance value « the cut-off value Equivocal: clinical sample absorbance value within 0.010 absorbance units of the cut-off value. These samples should be retested or the patient resampled.