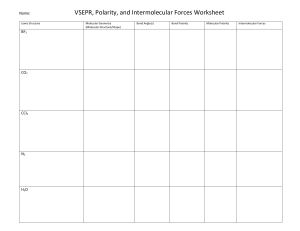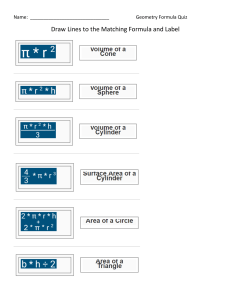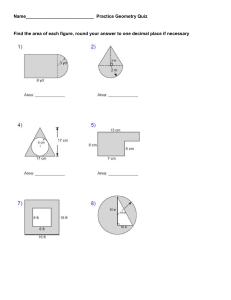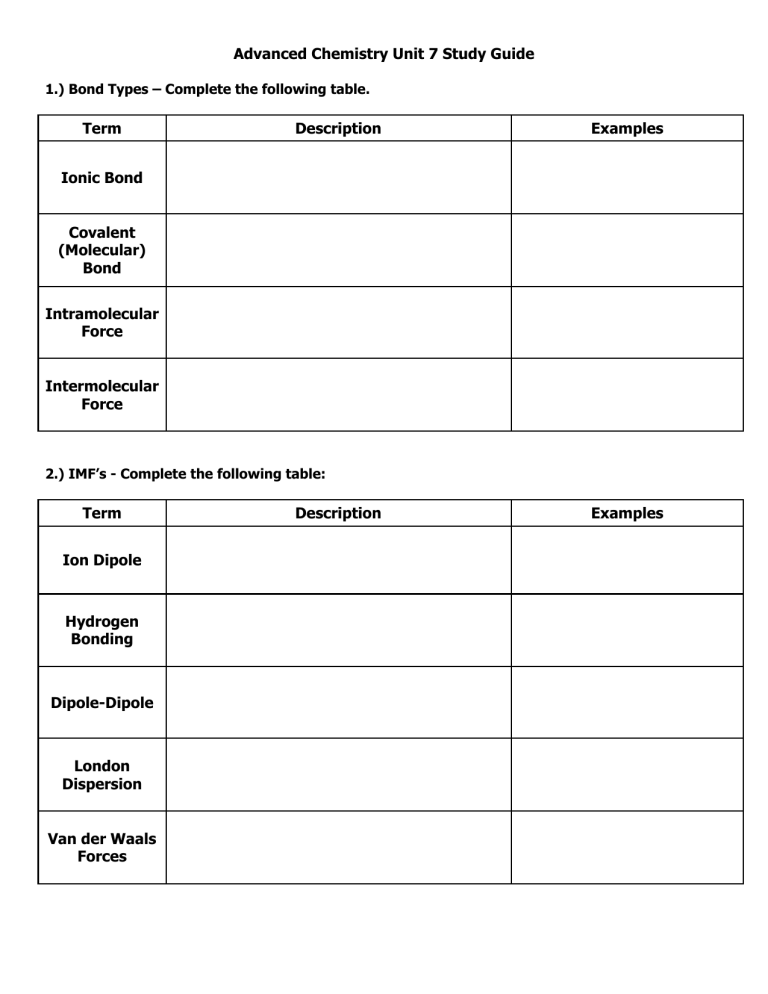
Advanced Chemistry Unit 7 Study Guide 1.) Bond Types – Complete the following table. Term Description Examples Ionic Bond Covalent (Molecular) Bond Intramolecular Force Intermolecular Force 2.) IMF’s - Complete the following table: Term Ion Dipole Hydrogen Bonding Dipole-Dipole London Dispersion Van der Waals Forces Description Examples 3.) VSEPR Theory a.) Describe the main idea of VSEPR Theory. b.) Describe what VSEPR Theory does. 4.) Lewis Structure and Geometry - Complete the following table: Term Central Atom Terminal Atom Lone Pairs (Non-Bonding Pairs, Unshared Pairs) Polarity Electronegativity Electron Domain Electron Domain Geometry Molecular Geometry Formal Charge Hybridization Description Examples 5.) Rank the IMFs (intermolecular forces) from strongest to weakest. 6.) In general, how do IMF’s affect the physical properties of a substance, such as boiling and melting point? 7.) Order the following substances from lowest to highest boiling point. Carbon dioxide, dihydrogen monoxide, phosphorus trihydride. 8.) Complete the following table: Chemical Name Chemical Formula Potassium Hydroxide N2O4 FeCl3 Ammonium Sulfate Carbon Tetrachloride SF6 Lead (IV) Oxide SiO2 Sodium Phosphide Mg2(PO4)3 Xenon Dichloride Ionic or Molecular 9.) Covalent Prefixes Number 1 2 3 4 5 6 7 8 9 10 Prefix 10.) Sigma and Pi Tell how many sigma and pi bonds are in each molecule. a) ______ sigma ______ pi b.) ______ sigma ______ pi c) ________ sigma _________ pi 11.) Complete the following tables: Lewis Structure TeBr6 CO2 NH3 ICl3 CF4 N2 Total Number of Electron Domains Electron Domain Geometry Hybridization Molecular Geometry Polarity Intermolecular Forces (list from strongest to weakest) Lewis Structure H2S PCl3 XeCl4 KrF2 HF HCN Total Number of Electron Domains Electron Domain Geometry Hybridization Molecular Geometry Polarity Intermolecular Forces (list from strongest to weakest) Lewis Structure BF3 BeCl2 PO43- ClO41- AsO33- ClF5 Total Number of Electron Domains Electron Domain Geometry Hybridization Molecular Geometry Polarity Intermolecular Forces (list from strongest to weakest)