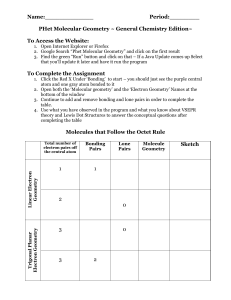
Name:_____________ Period:________ PHet Molecular Geometry ~ General Chemistry Edition~ To Access the Website: 1. Open Internet Explorer or Firefox 2. Google Search “Phet Molecular Geometry” and click on the first result 3. Find the green “Run” button and click on that – If a Java Update comes up Select that you’ll update it later and have it run the program To Complete the Assignment 1. Click the Red X Under ‘Bonding’ to start – you should just see the purple central atom and one gray atom bonded to it 2. Open both the ‘Molecular geometry’ and the ‘Electron Geometry’ Names at the bottom of the window 3. Continue to add and remove bonding and lone pairs in order to complete the table. 4. Use what you have observed in the program and what you know about VSEPR theory and Lewis Dot Structures to answer the conceptual questions after completing the table Trigonal Planar Electron Geometry Linear Electron Geometry Molecules that Follow the Octet Rule Total number of electron pairs off the central atom Bonding Pairs 1 1 2 0 3 3 Lone Pairs 0 2 Molecule Geometry Sketch Tetrahedral Electron Geometry 4 Bent 4 4 1 4 Concept Questions 1. What does VSEPR stand for? How did what you observed in the program demonstrate VSEPR theory in the geometry of different molecules? 2. Reset your molecule to a linear molecule with just two bonding groups. Try to push one of the bonding groups towards the other. Does what you see happen demonstrate VSEPR theory? 3. How are molecular geometries different from electron geometries? (What do electron geometries take into account that molecular geometries do not?) 4. Go back to the PHeT program and try adding double or triple bonds instead of single bonds. Does this seem to have an effect on the resulting structure? Explain. 5. For each of the following molecules or ions do the following: a. Write the formula if applicable b. Draw the Lewis Dot Structure c. Identify the number of both the bonding and lone electron pairs d. Determine the molecular geometry using the table that you created • Ammonia (NH3) • Water • Carbon Tetrachloride • Boron Trifluoride • Carbon Dioxide