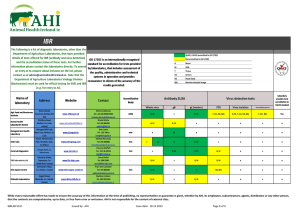
Th e dec om pos it ion
of c alc iumc ar bona t ei s o n l y
spontaneous
at high ternperatures.
Type6. CrHo(g; + Hr(S)
!
-
CrH6(9)
!Above a !certaintemperature
!
!this reaction
! wi
ceaseto be spontaneous.
*
Thereforeabove804'C the reactionwill be spontaneous.
$
$ Note:this calculationassumesthat the entropyvalue is
independentof temperature,
which is not strictlytrue.
{
Name!!
!
!
! N,*,----**d
!
!
IBQUESTIONS
- ENERGETICS
?
$
$
I
3.
1. Which statementaboutthis reactionis correct?
FerO,(s)+ 3CO(g) LHQ = +26.6 kj
A, 26.6 kJof energyare releasedfor everymole of Fe
reactecl
2Fe(s)+ 3CO,(9
8. 26.6 kl of energy are absorbeclfor ever1,nrole of Fe
reacted
C. 53.2 kl of energy are releasedt,orevery r-noieof Fe
reacted
D. 13.3 kJof energy:rreabsorbedfor everymole of Fe
reacted
2 , Wh enso lut ionsof HCI and NaO H ar e m ix edt h e
temperature
increases.
The reaction:
H* (aq )+ O H- ( aq) Hr O ( l)
A. is en dot her m ic
wit h a pos it iv eAHo.
What can be deducedabout the relativestabilityof the
reactantsand productsand the signof Al/e, from the
enthalpylevel diagramabove?
Relativestability
A. Productsmore stable
Sign of AHe
B. Productsmore stable
C. Re,rctanls
more stable
D. Reactants
more stable
+
B, is endothernric
with a negativeAHe.
C. is exothermicwith a positiveAHe.
D. is exothermicwith a negativeAHe.
lB questions
- Energetics
33
-----------------------------------------------------------------------------------------------------------------------------6. 2KHCo.(s)--g:-
4. Forthe reaction:
2 C(s)+ 2 Hr ( g)- C2Hq( 9
K2co3(s)+Cor(g)+ H2o(l)
AHI = 152. 3 L 1
+2H C l t a q )
lf LH|=-17 4 . 4k ) f or t he r eac t ion:
C2H2(g)+ Hr(g) -' CrHr(g)
what can lre said aboutthe value of AH] for the reactton
belorv?
2C(s)+Hr ( g)
- Cr Hr ( g)
A. AH: nrustbe negative.
B. AH: m us tbe a pos it iv enum bers m allert h a n 5 2 . 3 k J .
C. AH: m us tbe a pos it iv enum berlar gert han 52 . 3 k J .
D. No conclusioncan be made aboutAH! without the
va lue o f H f or Hr ( g) .
5. The enthalpychangesfor two differenthydrogenation
reactionsof CrH, are:
CrH, + H,
CrH, LHeCrH, + 2H., - CrHu LHI
Which expressionrepresents
the enthalpychangefor the
reactionbelow?
CrH,, + H,
A. AHf
+ AHre
B. AHi
- AH2o
CrHu
A,He = ?.
c. AH2e- AHi
D. -AHi
2 K C l ( a q )+ 2 C O , ( g )+ 2 H r O ( l )
This cycle may be usedto determineAHe for the
decompositionof potassiumhydrogencarbonate.
Which expressioncan be usedto calculateAHe?
A. AHe= LHi + LHi
C. AH' =+LHi , LH:z
B. AHe= LH? -LHi
D . a H o =L H ; - L H i
7. Use the bond energiesfor H-H (436 kJmol-r),
B r - B r ( 1 9 3 k J m o l r ) a n d H - B r ( 3 6 6 k Jm o l r ) t o
calculateAHe (in kJ mol r) fbr the reaction:
H r ( g )+B r r ( g )
A . 263
B . 103
-2HBr(g)
c. -1 03
D . 263
B. The averagebond enthalpyfor the C H bond is
4 1 2 k J m o l - 1. Wh i c h p r o c e s sh a sa n e n t h a l p yc ha ng e
c l o s e st o t h i s v a l u e ?
A . C H o ( g ) C ( s )+ 2 H r ( g )
B. CH4(9 C(8)+ 2Hz(g)
C. CHr(g) C(s)+ 4H(g)
D . C H 4 ( g ) - - C H r ( g )+ H ( g )
6
Ra
RA
Che
che
eithe
unit
reac
The
that
met
abs
cha
con
Dat
con
stat
Rate
use
The
use
dep
the p
follo
trA
I The
LH?
I thes
I mu
9. Whiclr of the changesbelow occursrvith the greatest
increasein entropy?
A. NarO(s )+ H r O ( l) * 2Na* ( aq)+ 2O H- ( aq )
B. NH3(8 )+ HCI ( g)
12. At 0 'C, the mixtureformedwhen the following reaction
r e a c h e se q u i l i b r i u mc o n s i s t sm o s t l yo f N r O r ( g ) .
2N02(g)-- NrOo(g)
I tne
aM
I
I As t
I not
I vor