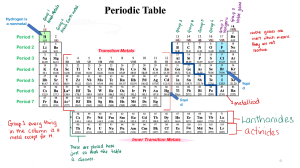
GRADE 09 SAT MODULE 17 LESSON 1 Content Lesson 1 Metals a) b) c) d) e) f) Properties of metal Metallic bonding The Alkali Metals The Alkaline earth Metals The transition Elements The inner transition elements g) Metals in Earth’s crust Properties of Metals • • • • • Conductor of heat and electricity Malleable Ductile Shiny Lusterous (Shiny) Metallic bonding ‘Metallic bond’ is a term used to describe the collective sharing of a sea of valence electrons between several positively charged metal ions. The factors that affect the strength of a metallic bond include: Total number of delocalized electrons. Magnitude of positive charge held by the metal cation. The Alkali Metals Li Na K Rb Cs Fr Alkali metals are shiny, malleable, ductile and good conductor of heat and electricity. These are soft metals. These are the reactive metals They don’t occur naturally in their elemental forms. These muse be stored in oil to prevent reaction with oxygen and water. The Alkali Metals Li Na K Rb Cs Fr These elements are important for our health. Most of our diet consists of these elements. Lithium compound are used to treat bipolar disorder. The lithium can regulate the chemical levels of our mental health. The Alkali Metals Li Na K Used in light detector sensors Rb Cesium is used in atomic clocks. Cs Francium is radioactive and extremely rare. Fr The Alkaline Earth Metals Be Mg Ca Sr Alkaline earth metals are shiny, malleable, ductile and good conductor of heat and electricity. These elements are reactive and are not found as free elements in nature Ba These metals have 2 valence electron and form positive ion. Ra Used to color fireworks. The Alkaline Earth Metals Be Mg • Its light and having good strength. Ca • Used in cars, Plane, space craft etc Sr • Present in chlorophyll (a pigment used in photosynthesis) Ba Ra The Alkaline Earth Metals Be Mg Ca Sr Ba Ra • It is seldom used as a free metal. • Marble statutes are made up of Calcium carbonates. • Many vitamins consists of Calcium. • Calcium phosphate helps make your bone strong. The Alkaline Earth Metals Be Mg Ca Sr Ba Ra • Barium sulfate (BaSO4) is used to diagnose some digestive disorders. • Radium is a radioactive metals. It is used to treat cancers. It is being replaced with more readily available radioactive elements. The Transition metals. The elements from group 3 to 12 are called transition metals. The Transition elements Fe Co Ni • These are called iron triads. • These are the magnetic elements and used in steel and other metal mixtures. • Iron is the second most abundant element in earth crust. • Nickel and cobalt are added to steel to give various properties. • Nickel is used to give shiny and protective coating to other metals. The Transition elements Cu Ag Au • These are called coinage metals because once these were used as a coin. • Used in athletic medals. • Copper is used in electrical wiring. • The compound like silver iodide (AgI) is used in photographic film. • Silver and gold are used in jewelry The Transition elements Zn Cd Hg • Zinc reacts with oxygen to form a thin protective layer of zinc oxide. • Cadmium also used in rechargeable batteries. • Mercury exists in liquid state and is used in thermometers, batteries etc. • Mercury is poisonous ` The inner transition Elements ` The atoms and Periodic table ` The inner transition Elements The inner transition elements are located between group 3 and 4 in period 6 and 7 The first row is called Lanthanide series. • Some of the element in this series with carbon make a compound used in movie lighting. • Compounds of Eu, Gd, Tb are used as a color phosphors. • Phosphors change UV light to visible light. The second row is called actinide series. ` The inner transition Elements The inner transition elements are located between group 3 and 4 in period 6 and 7 The second row is called actinide series. • All of the series elements are radioactive. • Thorium and uranium are the only actinides to found in earth crust. • Thorium is used in making the glass for camera lenses. • Uranium is used in nuclear reactors and in weapons. Metal in Earth’s Crust The surface of the earth consists of many compounds and few un-combined metals such as gold. Metals that are found in earth crusts are minerals. Minerals often found in ores. Ores are mixture of minerals, clay, and rocks and occurs naturally in earth crust. Metal in Earth’s Crust Metals are separated from its ore by different processes. The ores are heated and are called roasting. The ore is then refined to pure form. Late it can be mixed with other metals to form alloy. End of ELesson