Chemistry Textbook Excerpt: Periodicity, Electrochemistry, and More
advertisement

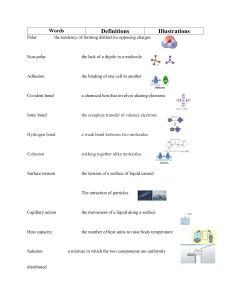
Periodicity: Dimitri Mendeleev – created first widely-accepted arrangements of elements Lanthanides and actinides and Sc and Y are known as rare earth elements An ionic bond is a chemical bond where one ion loses one or more electrons and transfers them to another ion. A covalent bond is a chemical bond where one or more electrons from one element leave that element and fill the outermost electron shell of the other element. When two or more metals form a compound, the resulting bond is known as a metallic bond. It is the metallic equivalent of a covalent bond. When the atoms in a covalent bond do not share the electrons equally, it is called a polar covalent bond. Electrochemistry: The octet rule states that all atoms (excluding H and He) are trying to get an octet (8) electrons. Electrochemical cells results in an exchange of electrons in a redox reaction. There are two main types of electrochemical cells. Voltaic Cells A voltaic, or galvanic, cell is composed of two metals connected by a salt bridge. It uses the electron exchange to generate the current. It consists of two half-cells, each of which contains a metal solution with that metal submerged in it. The cell in which oxidation occurs is called the anode, and the cell in which reduction occurs is called the cathode. Thus, electrons flow to the cathode, meaning that reduction is occurring at the cathode. This means that oxidation occurs at the anode. A porous barrier allows ions to flow from the anode compartment to the cathode compartment and vice versa, balancing the charge. The electrode compartments are called half-cells. Salt bridges may be used as an alternative to porous barriers. Electrolytic Cells Electrolytic cells use a current to decompose chemical compounds. One principle use of electrolytic cells is to electroplate objects such as nails and silverware. Coulombs is a measure of charge. Current is a measure of the flow of electrons. The SI unit of current is the ampere, expressed as C/s or coulombs per second. Coulombs = amperes × seconds Given the current run through an electrolytic cell and the time it is run, you can calculate the number of coulombs. There are 1.602E-19 coulombs in an electron. From the amount of coulombs you may calculate the number of electrons used to reduce. Say that you are electroplating copper onto a plate. Cu2++2e−→Cu Given the number of electrons used to reduce the copper ions, you may calculate the amount of Cu electroplated onto the plate. This is applicable to any electroplating situation. Electron Potential There are two different analogies for understanding electron potential or voltage. The emf of a cell, measured in volts, is the potential difference between the cathode and the anode of a cell. It tells you how much potential there is to do work. Electromotive means "causing electron motion". Standard Reduction (Half-Cell) Potentials Reduction Potentials tell you how much something "wants" to reduce. For example, Cu2+ (with a reduction reaction of Cu2++2e−→Cu) has a higher reduction potential then Fe2+ (with a reduction reaction of Fe2++2e−→Fe). This means that Cu2+ "wants" to reduce more then Fe2+. Like all potentials, reduction potentials are not absolute and have to be with respect to something. Standard Reduction Potentials, denoted Ered∘ are the reduction potential with respect to a reference reaction. This reference reaction is the reduction of H+. 2H++2e−→H2 An electrode designed to produce this half reaction is called a standard hydrogen electrode (SHE) or the normal hydrogen electrode (NHE). To calculate the emf of a cell, simply take the standard reduction potential of the cathode and subtract the standard reduction potential of the anode from it. Because electron potential measures potential energy per charge, the stoichiometric coefficients in the half-reactions do not affect the value of the standard reduction potential or the emf of the cell. The Nernst equation at a standard temperature for the above equation is shown below. Aqueous Solutions There are some exceptions to these rules, including: • AgF is soluble • PbCl2, PbBr2, PbI2 are mildly soluble at high temperatures • Ag2SO4 is mildly soluble • Li2CO3, Li3PO4, LiF are insoluble Equilibrium Constant Take this reaction: aA+bB→cC+dD The equilibrium constant is equal to: k=[C]c∗[D]d[A]a∗[B]b Where all of the concentrations are the concentrations at equilibrium and where solids are excluded. Solubility Product Equilibrium Constant The solubility product equilibrium constant (Ksp) is equal to [A+][B−] for the following reaction: AB→A++B− Unsaturated - Unsaturated solutions are solutions where the Ksp has not yet been reached. Saturated - Saturated solutions are solutions where the Ksp has been reached. Super Saturated- Super Saturated solutions are where the Ksp has been reached and gone over. These solutions can be achieved by heating up a solvent (heat causes the Ksp to increase), adding a solute, and then letting the solution cool. Miscible- Two things are considered miscible when they can be mixed uniformly in any quantities. The equilibrium expression for the autoionization of water is: Kw=[H+][OH−] pH - From the equilibrium constant for water, the pH of a solution can be calculated. pH (potential hydrogen) is equal to −log[H3O+], the negative logarithm of the concentration of H3O The basic idea of the common ion effect is that if a solute is added that contains an ion already in solution, it will affect the equilibrium of the solution. For example, if hydrochloric acid (HCl) is added to a solution of NaCl, this will drive the reaction (dissociation of solid NaCl) toward the reactant, causing NaCl to precipitate out of solution. The solubility product, Ksp, is an equilibrium constant that expresses how much a compound dissolves in solution. Because these are examples of heterogeneous equilibria - a solid is involved in the reaction - the solubility product is equal to the concentration of the first ion to the power of its coefficient times the concentration of the second ion to the power of its coefficient. For example, for the dissociation of barium sulfate (BaSO 4), the solubility product is equal to [Ba2+][SO42−]. Thermodynamics 1st law of thermodynamics – energy is neither created nor destroyed 2nd law of thermodrynamics - In an isolated system, a natural process is spontaneous if it leads to an increase in entropy 3rd law of thermodynamics - The entropy of a perfect crystal is zero when the temperature of the crystal is absolute zero. Enthalpy: heat content H=E+PV. Entropy: disorder in system ΔS=ΣS(products)−ΣS(reactants) Gibbs free energy: spontentaity based on enthalpy and entropy ΔG=ΔH−TΔS Acid/Bases Arrhenius acids are defined to be chemicals that, when put in water, produce hydronium (H3O+) ions. Similarly, Arrhenius bases produce hydroxide (OH−) ions in aqueous solution. Bronsted-Lowry acids are defined to be chemicals that donate protons (H+), while Bronsted bases accept protons. Similar to acids, strong Bronsted-Lowry bases are bases that almost completely disassociate in water. Examples include alkali hydroxides such as: LiOH,NaOH,KOH,RbOH, and CsOH. For the following reaction: HA+H2O⇌H3O++A− The acid dissociation constant (Ka) is equal to ([H3O+][A−])/[HA] Similarly, we can also define a base dissociation constant Kb. For the reaction B+H2O⇌BH++OH− The base dissociation constant (Kb) is equal to ([BH+][OH−])/[B] You will often see Ka and Kb expressed as pKa and pKb, where: pKa=−log(Ka) pKb=−log(Kb) Relationship between Ka and Kb [H+][NH3][NH4+]∗[NH4+][OH−][NH3]=[H+][OH−] This method works for all acids and bases. Thus, Ka∗Kb=Kw Buffer pH The pH of a buffer is determined by a few factors: the K a constant of the weak acid, and the ratio of the weak base to the weak acid in the solution. This equation is known as the Henderson-Hasselbalch equation. pH=pKa+log([B][A]) The pH of a buffer can be controlled by altering the ratio of base to acid in the solution, as the pKa value is a quality of the acid chosen for the buffer. Titrations Gas Laws Boyles Law P1V1=P2V2 where P is the pressure of the gas and V is the volume of the gas. Charles’s Law V1/T1=V2/T2 where V is the volume of the gas and T is the temperature of the gas. Gay Lussacs Law P1/T1=P2/T2 Combined Gas Law P1V1/T1=P2V2/T2 Ideal Gas Law PV=nRT • • • 8.314 kPa L/moles K AND 8.314 J/moles K 0.08206 atm L/moles K 62.36 mmHg L/moles K. Dalton's Law Ptotal=P1+P2+...+Pn P1=χ1Ptotal The mole fraction can be calculated by this formula: χ1=moles of gas 1moles of mixture KEavg=3RT/2 vrms=rt3RT/M