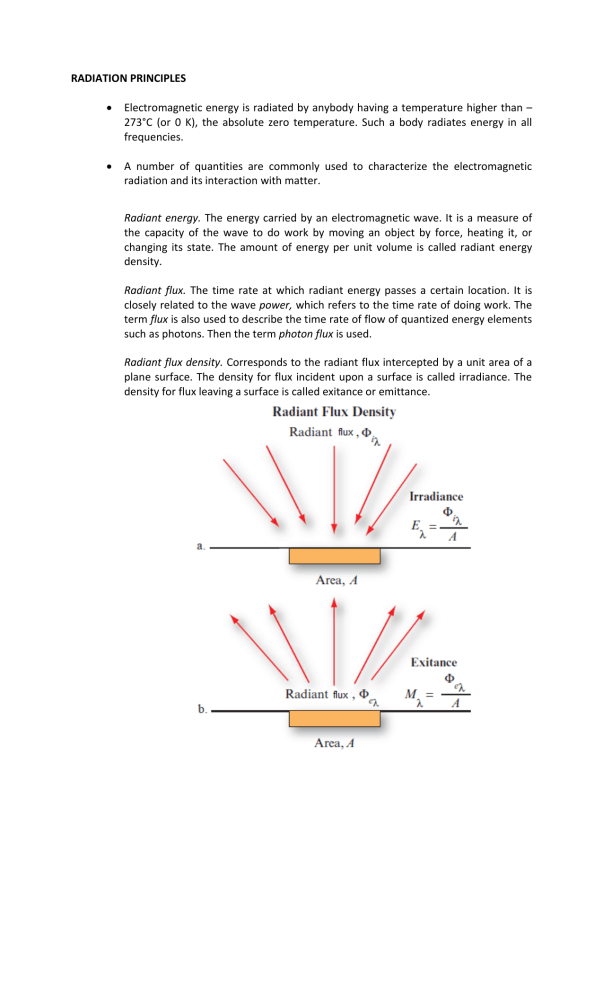
RADIATION PRINCIPLES Electromagnetic energy is radiated by anybody having a temperature higher than – 273°C (or 0 K), the absolute zero temperature. Such a body radiates energy in all frequencies. A number of quantities are commonly used to characterize the electromagnetic radiation and its interaction with matter. Radiant energy. The energy carried by an electromagnetic wave. It is a measure of the capacity of the wave to do work by moving an object by force, heating it, or changing its state. The amount of energy per unit volume is called radiant energy density. Radiant flux. The time rate at which radiant energy passes a certain location. It is closely related to the wave power, which refers to the time rate of doing work. The term flux is also used to describe the time rate of flow of quantized energy elements such as photons. Then the term photon flux is used. Radiant flux density. Corresponds to the radiant flux intercepted by a unit area of a plane surface. The density for flux incident upon a surface is called irradiance. The density for flux leaving a surface is called exitance or emittance. Solid angle. The solid angle _ subtended by area A on a spherical surface is equal to the area A divided by the square of the radius of the sphere. Radiant intensity. The radiant intensity of a point source in a given direction is the radiant flux per unit solid angle leaving the source in that direction. Radiance. The radiant flux per unit solid angle leaving an extended source in a given direction per unit projected area in that direction. If the radiance does not change as a function of the direction of emission, the source is called Lambertian. A piece of white matte paper, illuminated by diffuse skylight, is a good example of a Lambertian source. Hemispherical reflectance. The ratio of the reflected exitance (or emittance) from a plane of material to the irradiance on that plane. Hemispherical transmittance. The ratio of the transmitted exitance, leaving the opposite side of the plane, to the irradiance. Planck’s law The spectral radiance emitted by a blackbody as a function of wavelength and temperature T is given by where Planck’s constant h is 6.626 10 , Boltzmann’s constant and the units of ( ) are typically expressed as / / / 1.381 / . 10 / , Wavelength, Frequency and Energy (Q): The energy (in Joules) released from a radiating body in the form of a vibrating photon (quantum) can be computed as: =ℎ where: = ℎ () ℎ= ( 6.626 10 ) Black Body Radiation The blackbody is a hypothetical entity because in nature all objects reflect at least a small proportion of the radiation that strikes them and thus do not act as perfect reradiators of absorbed energy. It emits EMR at all wavelengths if its temperature is above 0 Kelvin (absolute 0) Properties: 1) Absorbs all radiation incident on it (perfect absorber). 2) Emits the maximum amount of radiation at all temperatures (perfect radiator). –Contrasts Contrasts with white body, theoretical body that reflects all incident ra radiation. EM Energy Emission and Temperature • All matter at temperatures above absolute zero (0 K) continuously emits EMR • Both the sun and Earth's surface behave as blackbodies. An object that absorbs and emits all possible radiation at 100 percent efficie efficiency ncy is called a blackbody. • Energy emitted from an object is a function of its surface temperature (Planck’s law) • The emitted spectrum is also a function of its temperature. –The The higher the temperature of a body, the more radiation it emits at every wavelen wavelength RADIATION LAWS FOR UNDERSTANDING ELECTROMAGNETIC RADIATION Kirchoff’s Law and Emissivity • States tates that the ratio of emitted radiation to absorbed radiation flux is the same for all blackbodies at the same temperature.It relates the real performance to that expected for a black body: • It relates the real performance to that expected for a black body: = = = Emissivity vity for natural body ( )- the ratio between the emittance of a given object and that of a blackbody at the same temperature • Most objects are not blackbodies, they emit less than the maximum energy for their temperature (“gray bodies”) *A grey body has an emissivity less than 1 but is constant at all wavelengths. -Water Water is a good approximation of a black body (grey body) Stefan–Boltzmann Boltzmann law • Defines efines the relationship between the total emitted radiation (W) (often expressed in watts · cm–2) and temperature (T) (absolute temperature, K): = where: = Stefan– Boltzmann constant (5.6697 × 10 watts. m . K ) • The he law states that hot blackbodies emit more energy per unit area than do cool blackbodies. Wien’s displacement law • Specifies pecifies the relationship between the wavelength of radiation emitted and the temperature of a blackbody: = 2,897.8 where λ is the wavelength at which radiance is at a maximum and T is the absolute temperature (K). • As blackbodies become hotter, the wavelength of maximum emittance shifts to shorter wavelengths. • The wavelength at which maximum energy is emitted is the ‘color’ of emitting object called the brightness temperature.






