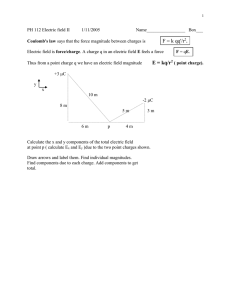
General Physics (2) Dr. Hasan Maridi Assistant Professor of Theoretical Nuclear Physics at Taiz University, Yemen https://sites.google.com/site/hasanmaridi Chapter 1 - Electric Fields References: 1-Physics for Scientists and Engineers (with PhysicsNOW and InfoTrac), Raymond A. Serway - Emeritus, James Madison University , Thomson Brooks/Cole © 2004, 6th Edition, 1296 pages. 2- Power point slides of Serway book (Physics for Scientists and Engineers) from Cengage Learning Company (http://www.cengage.com). Electricity and Magnetism, Some History Chinese Documents suggest that magnetism was observed as early as 2000 BC Greeks Electrical and magnetic phenomena as early as 700 BC Experiments with amber and magnetite 1600 William Gilbert showed electrification effects were not confined to just amber. The electrification effects were a general phenomena. 1785 Charles Coulomb confirmed inverse square law form for electric forces Electricity and Magnetism, More History 1819 Hans Oersted found a compass needle deflected when near a wire carrying an electric current. 1831 Michael Faraday and Joseph Henry showed that when a wire is moved near a magnet, an electric current is produced in the wire. 1873 James Clerk Maxwell used observations and other experimental facts as a basis for formulating the laws of electromagnetism. Unified electricity and magnetism Electric Charges There are two kinds of electric charges Called positive and negative Negative charges are the type possessed by electrons. Positive charges are the type possessed by protons. Charges of the same sign repel one another and charges with opposite signs attract one another. The rubber rod is negatively charged.The glass rod is positively charged. The two rods will attract. The rubber rod is negatively charged. The second rubber rod is also negatively charged. The two rods will repel. Conservation of Electric Charges Electric charge is always conserved in an isolated system. A glass rod is rubbed with silk. Electrons are transferred from the glass to the silk. Each electron adds a negative charge to the silk. An equal positive charge is left on the rod. Conductors Electrical conductors are materials in which some of the electrons are free electrons. Free electrons are not bound to the atoms. These electrons can move relatively freely through the material. Examples of good conductors include copper, aluminum and silver. When a good conductor is charged in a small region, the charge readily distributes itself over the entire surface of the material. Insulators Electrical insulators are materials in which all of the electrons are bound to atoms. These electrons can not move relatively freely through the material. Examples of good insulators include glass, rubber and wood. When a good insulator is charged in a small region, the charge is unable to move to other regions of the material. Semiconductors The electrical properties of semiconductors are somewhere between those of insulators and conductors. Examples of semiconductor materials include silicon and germanium. Semiconductors made from these materials are commonly used in making electronic chips. The electrical properties of semiconductors can be changed by the addition of controlled amounts of certain atoms to the material. Charging by Induction Charging by induction requires no contact with the object inducing the charge. Assume we start with a neutral metallic sphere. The sphere has the same number of positive and negative charges. Charging by Induction, 2 B: A charged rubber rod is placed near the sphere. It does not touch the sphere. The electrons in the neutral sphere are redistributed. C: The sphere is grounded. Some electrons can leave the sphere through the ground wire. Charging by Induction, 3 D: The ground wire is removed. There will now be more positive charges. The charges are not uniformly distributed. The positive charge has been induced in the sphere. E: The rod is removed. The electrons remaining on the sphere redistribute themselves. There is still a net positive charge on the sphere. The charge is now uniformly distributed. Note the rod lost none of its negative charge during this process. Charge Rearrangement in Insulators A process similar to induction can take place in insulators. The charges within the molecules of the material are rearranged. The proximity of the positive charges on the surface of the object and the negative charges on the surface of the insulator results in an attractive force between the object and the insulator. Coulomb’s Law Charles Coulomb 1736 – 1806 French physicist Major contributions were in areas of electrostatics and magnetism Also investigated in areas of Strengths of materials Structural mechanics Ergonomics Charles Coulomb measured the magnitudes of electric forces between two small charged spheres. The force is inversely proportional to the square of the separation r between the charges and directed along the line joining them. The force is proportional to the product of the charges, q 1 and q 2, on the two particles. The electrical force between two stationary point charges is given by Coulomb’s Law. Point Charge The term point charge refers to a particle of zero size that carries an electric charge. The electrical behavior of electrons and protons is well described by modeling them as point charges. The force is attractive if the charges are of opposite sign. The force is repulsive if the charges are of like sign. Quantization of Electric Charges The electric charge, q, is said to be quantized. q is the standard symbol used for charge as a variable. Electric charge exists as discrete packets. q = Ne N is an integer e is the fundamental unit of charge |e| = 1.6 x 10-19 C Electron: q = -e Proton: q = +e Coulomb’s Law, Equation Mathematically, Fe k e q1 q 2 r2 The SI unit of charge is the coulomb ©. k e is called the Coulomb constant. k e = 8.9876 x 109 N.m2/C2 = 1/(4πe o) e o is the permittivity of free space. e o = 8.8542 x 10-12 C2 / N.m2 Example: The Hydrogen Atom The electron and proton of a hydrogen atom are separated (on the average) by a distance of approximately 5.3 x10-11 m. Find the magnitudes of the electric force and the gravitational force between the two particles. Solution From Coulomb’s law, we find that the magnitude of the electric force is Using Newton’s law of universal gravitation and Table 23.1, we find Thus, the gravitational force between charged atomic particles is negligible when compared with the electric force. Coulomb's Law, Notes Remember the charges need to be in coulombs. e is the smallest unit of charge. except quarks e = 1.6 x 10-19 C So 1 C needs 6.24 x 1018 electrons or protons Typical charges can be in the µC range. The electron and proton are identical in the magnitude of their charge, but very different in mass. The proton and the neutron are similar in mass, but very different in charge. Vector Nature of Electric Forces In vector form, F12 ke q1q2 rˆ12 2 r r̂12 is a unit vector directed from q1 to q2. The like charges produce a repulsive force between them. Electrical forces obey Newton’s Third Law. The force on q1 is equal in magnitude and opposite in direction to the force on q2 F21 F12 With like signs for the charges, the product q1q2 is positive and the force is repulsive. With unlike signs for the charges, the product q1q2 is negative and the force is attractive force between them. The sign of the product of q1q2 gives the relative direction of the force between q1 and q2. Multiple Charges The resultant force on any one charge equals the vector sum of the forces exerted by the other individual charges that are present. Remember to add the forces as vectors. The resultant force on q 1 is the vector sum of all the forces exerted on it by other charges. For example, if four charges are present, the resultant force on one of these equals the vector sum of the forces exerted on it by each of the other charges. F1 F21 F31 F41 Zero Resultant Force, Example Where is the resultant force equal to zero? Three point charges lie along the x axis as shown in Figure. The positive charge q 1 = 15.0 C is at x ! 2.00 m, the positive charge q 2 = 6.00 C is at the origin, and the resultant force acting on q3 is zero. What is the x coordinate of q 3? Solution Because q 3 is negative and q 1 and q 2 are positive, the forces F13 and F23 are both attractive. From Coulomb’s law, F13 and F23 have magnitudes For the resultant force on q 3 to be zero, F23 must be equal in magnitude and opposite in direction to F13. we have Solving this quadratic equation for x, we find that the positive root is x= 0.775 m There is also a second root, x =-3.44 m. This is another location at which the magnitudes of the forces on q3 are equal, but both forces are in the same direction at this location.. Electric Field – Introduction The electric force is a field force. Field forces can act through space. The effect is produced even with no physical contact between objects. Faraday developed the concept of a field in terms of electric fields. An electric field is said to exist in the region of space around a charged object. This charged object is the source charge. When another charged object, the test charge, enters this electric field, an electric force acts on it. The electric field is defined as the electric force on the test charge per unit charge. The electric field vector, E , at a point in space is defined as the electric force acting on a positive test charge, q o, placed at that point divided by the test charge: F E qo Electric Field, Notes is the field produced by some charge or charge distribution, separate from the test charge. E The existence of an electric field is a property of the source charge. The presence of the test charge is not necessary for the field to exist. The test charge serves as a detector of the field. The direction of E is that of the force on a positive test charge. The SI units of E are N/C. We can also say that an electric field exists at a point if a test charge at that point experiences an electric force. Relationship Between F and E Fe qE This is valid for a point charge only. One of zero size For larger objects, the field may vary over the size of the object. If q is positive, the force and the field are in the same direction. If q is negative, the force and the field are in opposite directions. Remember Coulomb’s law, between the source and test charges, can be expressed as Fe ke qqo rˆ 2 r Then, the electric field will be E Fe q ke 2 rˆ qo r More About Electric Field Direction a) q is positive, the force is directed away from q. b) The direction of the field is also away from the positive source charge. c) q is negative, the force is directed toward q. d) The field is also toward the negative source charge. Electric Fields from Multiple Charges At any point P, the total electric field due to a group of source charges equals the vector sum of the electric fields of all the charges. qi E ke 2 rˆi i ri Example: Electric Field Due to Two Charges A charge q 1 = 7.0 C is located at the origin, and a second charge q 2 = 5.0 C is located on the x axis, 0.30 m from the origin. Find the electric field at the point P, which has coordinates (0, 0.40) m. Solution First, let us find the magnitude of the electric field at P due to each charge. The fields E1 due to the 7.0 C charge and E2 due to the 5.0 C charge are shown in Figure 23.14. Their magnitudes are The vector E1 has only a y component. The vector E2 has an x component given by E 2 cos = 3/5 E 2 and a negative y component given by "E 2 sin = -4/5 E . Hence, we can express the vectors as From this result, we find that E makes an angle of 66° with the positive x axis and has a magnitude of 2.7 x 105 N/C. 2 Electric Field – Continuous Charge Distribution The distances between charges in a group of charges may be much smaller than the distance between the group and a point of interest. In this situation, the system of charges can be modeled as continuous. The system of closely spaced charges is equivalent to a total charge that is continuously distributed along some line, over some surface, or throughout some volume. For the individual charge elements E ke q rˆ 2 r Because the charge distribution is continuous E ke lim qi 0 and 𝑄 = i 𝑑𝑞 qi dq ˆ r k rˆ i e 2 2 ri r Charge Densities Volume charge density: when a charge is distributed evenly throughout a volume ρ ≡ Q / V with units C/m3 Surface charge density: when a charge is distributed evenly over a surface area σ ≡ Q / A with units C/m2 Linear charge density: when a charge is distributed along a line λ ≡ Q / ℓ with units C/m Amount of Charge in a Small Volume If the charge is nonuniformly distributed over a volume, surface, or line, the amount of charge, dq, is given by For the volume: dq = ρ dV For the surface: dq = σ dA For the length element: dq = λ dℓ Electric Field Lines The line has a direction that is the same as that of the electric field vector. The number of lines per unit area through a surface perpendicular to the lines is proportional to the magnitude of the electric field in that region. The density of lines through surface A is greater than through surface B. The magnitude of the electric field is greater on surface A than B. The lines at different locations point in different directions. This indicates the field is nonuniform. Electric Field Lines, Positive Pointand negative Charges For a positive point charge: The field lines radiate outward in all directions. In three dimensions, the distribution is spherical. The lines are directed away from the source charge. A positive test charge would be repelled away from the positive source charge. For a negative point charge: The field lines radiate inward in all directions. The lines are directed toward the source charge. A positive test charge would be attracted toward the negative source charge. Electric Field Lines – Rules for Drawing The lines must begin on a positive charge and terminate on a negative charge. No two field lines can cross. The number of lines drawn leaving a positive charge or approaching a negative charge is proportional to the magnitude of the charge. Field lines are not material objects, they describe the electric field. Electric Field Lines – Dipole The charges are equal and opposite. The number of field lines leaving the positive charge equals the number of lines terminating on the negative charge. Electric Field Lines – Like Charges The charges are equal and positive. The same number of lines leave each charge since they are equal in magnitude. Motion of Charged Particles When a charged particle is placed in an electric field, it experiences an electrical force. If this is the only force on the particle, it must be the net force. The net force will cause the particle to accelerate according to Newton’s second law. Fe qE ma If the field is uniform, then the acceleration is constant. The particle under constant acceleration model can be applied to the motion of the particle. The electric force causes a particle to move according to the models of forces and motion. If the particle has a positive charge, its acceleration is in the direction of the field. If the particle has a negative charge, its acceleration is in the direction opposite the electric field. Electron in a Uniform Field, Example An electron enters the region of a uniform electric field as shown in Figure, with E = 200 N/C. Find the acceleration of the electron while it is in the electric field. Solution The charge on the electron has an absolute value of 1.60 x 10-19 C, and me = 9.11 x 1031 kg. Therefore,