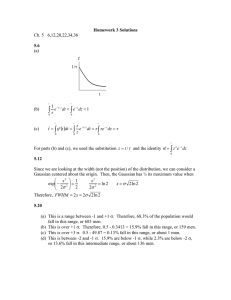
Diffusion and Phase Transformation
簡 朝 和
•
Course Outline
•
1.
(a)
(b)
(c)
(d)
(e)
•
•
•
•
•
•
•
•
•
•
Diffusion
2.
Diffusion equation solutions – steady and transient states
Diffusion with moving boundary
Diffusion in heterogeneous systems – controlling kinetics
Atomic theory of diffusion
Diffusion in dilute and concentrated solutions
Phase Transformation
(a) Nucleation - homogeneous and heterogeneous
(b) Growth with and without composition change
(c) Overall transformation kinetics
(d) Spinodal decomposition
•
Textbooks and References
•
•
•
•
•
•
•
•
•
1. P.G. Shewmon, Diffusion in Solids, 2nd ed., McGraw-Hill
2. D.R. Poirier and G.H. Geiger, Transport Phenomena in Materials Processing, 2nd ed., TMS publication, 1994.
3. J. Crank, Mathematics of Diffusion, 2nd ed., Oxford University Press, 1975.
4. H.S. Carslaw and J.C. Jaeger, Conduction of Heat in Solids, 2nd, ed., Oxford University Press, 1959.
5. A.K. Jena and M.C. Chaturvedi, Phase Transformations in Materials, Prentice Hall, NJ, 1992.
6. D.A. Porter, K.E. Easterling and Y. Sherif, Phase Transformations in Metals and Alloys, 3rd ed., CRC
Press, 2009.
7. J.W. Christian, The Theory of Transformations in Metals and Alloys, Pergamon Press, 1975.
8 R. W. Balluffi, S. M. Allen and W. C. Carter, Kinetics of Materials, Wiley, 2005.
•
Grading
•
•
•
•
Quizzes (2)
30%
Midterm test
25%
Final test
40%
Class attendance –random quizzes 5%
No aid sheet, No open book and No take home
Thermodynamics vs. Kinetics
• Thermodynamics:
– To study the direction of a reaction, or if a reaction can
take place. (ΔG<0)
– To study the equilibrium states in which state variables of
a system do not change with time.
• Kinetics:
– To study the rates and paths of a reaction adopted by
the systems approaching equilibrium.
– To study the rate-limiting steps of a reaction
– To study the controlling factors of the rate-limiting steps
Input
Kinetic Processes
• Rate-limiting steps
• Controlling factors
Output
Thermodynamics vs. Kinetics
Q
G1
ΔG
G2
Initial
State
Activated
State
Final
State
Reaction Rate α (Kinetic factor) x (Thermodynamic factor)
* Kinetic factor relates to Q (activation energy), while the
thermodynamic factor relates to the driving force, ΔG=G2-G1.
* The thermodynamic factor decides the direction of a reaction,
while the kinetic factor, the rate of reaction.
Kinetic theory: The reaction rate is proportional to the probability
to reach activated state that follows the Arrhenius
rate equation, exp(-Q /RT).
* The activation energy (Q) can be obtained from the slope of curve
plotted as ln (reaction rate) vs. 1/T
Example: For diamond growth by CVD from reaction of methane and hydrogen
- Q/R
Kinetic factor
Free Energy
adding catalysts.
increased by changing temperature or
Activation energy (Q)
without catalyst
Reactants
Activation energy (Q)
with catalyst
ΔG<0
Products
Progress of Reaction
Examples: (1) N2 + 3H2 = 2NH3 using iron as a catalyst
(2) 2CO + 2NO = 2CO2 + N2 using Pt and Rh as
catalysts for catalytic converters used in automobile
Examples: Thermodynamically favorable but
kinetically unfavorable phase changes
(1) Is a diamond forever?
Diamond
Diamond
Free Energy
Pressure (Pa)
1011
Graphite
Liquid
109
Graphite
7
10
Vapor
0
2000
4000
Temperature (K)
6000
Very large Q
Diamond
ΔG=-2.9KJ/mol
Graphite
Progress of Reaction
(2) Crystallization of glasses
1 h
Supercooled
liquid
Cooling Rate
2 h
Shrinkage due
to freezing
8 50m
h
Pseudowollastonite
(Ca,Ba,Sr)SiO3
Cristobalite SiO2
CaO-SrO-BaO-B2O3-SiO2 glass-ceramics annealed at 875oC
Diffusion driven by decrease in chemical potential
* Down-hill diffusion
2
1
A
B
Free energy of diffusion couple = G3
Diffusion taking place to homogenize
to obtain G4
G2
μ2A
G3
G1
μ1B
μ1B>μ2B B diffusing from (1)→(2)
G4
μ2A>μ1A A diffusing from (2)→(1)
Down-hill Diffusion
μ1A
μ2B
A
2
1
B
* Up-hill Diffusion
2
1
A
Free energy of diffusion couple = G3
Diffusion taking place to homogenize
to obtain G4
B
μ2B μ B<μ B B
1
2
diffusing from (2)
to (1)
μ 1A
μ2A<μ1A A diffusing from (1)
G2
G3
μ 1B
G4
μ 2A
A
2
to (2)
G1
1
B
Up-hill Diffusion
C
(not
)
x
x
J Cv C ( B F ) C B ( )
x
B : Mobility, F : Force
Driving force
Diffusion:
Process by which matter is transported through matter as a result of
molecular motions
time
General scheme for transport phenomena
Flux α Driving force α Gradient in potential
Matter
J
Heat
q
Electricity I
α dC/dx
α dT/dx
α dψ/dx
α Concentration potential
α Temperature potential
α Electrical potential
J DC ( Fick ' s Law)
D: diffusivity
q k T ( Fourier ' s Law)
k: thermal conductivity
I (Ohm ' s Law)
: electrical conductivity
(1)
Fick’s First Law
(2)
Δx
Rate of particles reaching surface (2):
number of particles/time =1
(a)
(b)
To increase the rate:
(a) Reducing thickness (Δx)
(b) Increasing number of particles (ΔC)
(c)
(c) Increasing area (A)
(d) Reducing particle size (D)
(e) Increasing temperature (D) (D=Doexp(-Q/RT))
C
A
x
n
C
v
J ( F lu x )
A
x
t A
C
D
(D : D iffu s iv ity )
x
v ( R a te )
n
t
(d)
Fick’s First Law:
Species migrates from a region of high concentration to a region of
low concentration;in general the rate of diffusion is proportional to
the concentration gradient
C
J D
x
*
Flux (J) : Mass/(area‧time), e.g., g/(cm2‧sec)
*
Minus (-): Matter moves from high to low concentration.
*
Diffusivity (D): Diffusivity related to atomic mobility and crystal
structure, e.g., cm2/sec (independent of
concentration gradient)
C
): Gradient in ”Mass Potential,”
* Concentration gradient (
x
e.g., g‧cm-3/cm
Magnitude of Diffusivity
Species
Diffusivity (cm2/sec)
Solids (metals near M.P.)
10-8
Elemental Semiconductors
10-12
Liquids
10-4-10-5
Gases
10-1
Note: Liquids and gases in liquids and gases generally dominated
by convection flow, rather than diffusion.
Diffusion << Convection
Diffusion
(Slower)
Convection
(Faster)
Steady State:
H
Concentration at a given point
is invariant with time
C C ( x)
Concentration
i.e.,
Ceq
C
( )x 0
t
Steady
State
t=∞
Time
J≠J(x,t) when area is fixed
Steady State Solution : C( x ) = A + Bx = Co -
Co
x
L
Transient
State
X=0
Key: to describe C(x,t) quantitatively
X=L
Equilibrium State: No Flux
C
( )t 0 ( )t
x
x
Steady State:
Constant flux if the area is fixed.
C
( )x 0 ( )x
t
t
μ: Chemical Potential
RT ln (a ) RT ln( X )
(
x
) t RT (
ln( a )
x
) t RT (
ln( X )
x
)t
Steady State:
J1
C
C
J
J1
No Depletion or Accumulation
area is fixed
( C t ) x 0
Under any conditions
2
( C
(J
x 2
x
)t 0
)t 0
t
x
x
C C
when D is constant
x x
Fick’s Second Law
Transient State: C=C(x,t), or J=J(x,t)
(
C
)x 0
t
accumulation or depletion of concentration exists
A (area)
JJX+ΔX
x+Δx
JJXx
Mass Conservation
X
x
X+ΔX
x+Δx
m
C
J x A J x x A
A x
t
t
(A is fixed)
Flux · Area = Rate of change of concentration · volume
(g cm-2sec-1· cm2 = g cm-3sec-1 · cm3)
From Taylor Series
J x x
J
2 J dx 2
Jx
dx 2
........
x
x 2!
J
C
dx) A
A x
x
t
J
C
dx A
A x
x
t
J x A (J x
Fick’s Second Law (cont.)
C J
C
)
( D
t x x
x
2C C D
D 2
x
x x
2C C D C
D 2
(
)
x
x C x
when
D
D D(C )
0
C
C
2C
D 2
t
x
D D(C )
Steady State
C
Fick’s Second Law
Linear partial differential equation
Solutions are additive
Solutions require initial and boundary conditions
x
Transient State:
J1
J2
Depletion or Accumulation
C
t
(C t ) x 0
C
)t 0
x
(J x )t 0
x
2
( C
If J2<J1
Accumulation
x 2
J
C J
2C
D 2
t x
x
Steady State
Cartesian coordinate in one dimension
C
2C
( )x D 2 0
t
x
if C= f(r)
Cylindrical coordinate
D C
C 1 C
(r
) D( 2
)0
r
r r r
r r
2
Spherical coordinate
D 2 C
C 2 C
(r
) D( 2
)0
2
r
r
r r
r r
2
(D is constant)
Application of Steady State
k
C CO2
2CO
- Infinite cylinder
k[CO]2
ac
; or
[CO2 ]
- Decarburization or Carburization
in single-phase Fe-C alloy
- No phase transformation involved
- Steady state reached ( ( Ct ) x 0)
- Diffusion controlling process
Carburization Gas
- To find D(C) in Fe-C alloy
Decarburization Gas
(Equilibrium concentrations reached on both sides)
k
2H 2 C
CH 4
k[CH 4 ]
ac
PH 2 2
Infinite Cylinder (area is not fixed)
For steady radial diffusion through the wall of the hollow cylinder, the Laplace equation in
cylindrical coordinate is
Steady State : (
C
)x 0
t
1 C
1 C C
(r
) 2
2 0
2
r r r
r
Z
2
Cylindrical coordinate :
2
r1
r2
If the concentration depends only on the radial coordinate
1 C
2C 1 C
(r
)0 2 +
=0 C (r ) A B ln r
r
r r r
r r
B.C.: C(r1, t)= C1, C(r2, t)= C2
r )
ln(
C C2
r2
C1 C2 ln( r1 )
r2
C1
C2
A plot of C versus ln(r) should be a straight line if
diffusion is the rate-controlling step and D is constant.
Diffusion of carbon in γ-Fe
Carbon content
Experimental data
Steady State : (
Not linear
D=D(C)
The above analysis
can not be applied
-log(r)
C
)x 0
t
The quantity of carbon passing through the tube per unit time (j) is constant and
independent of r:
j (mass/time) = 2πrlJr
(Flow Rate)
Area: 2πrl is not constant
r: Radius
l: Length of cylinder
Jr: Local flux
Jr D
C
r
j 2 rl ( D
C
C
C
j
r
)
r
r
ln(r )
2 lD
Steady State (diffusion area is not fixed)
C
C
( )x D 2 0
t
x
2
m1
m2
j1
j2
t
t
2 r1lJ r1 2 r2lJ r 2
r1 J r1 r2 J r 2
r1 r2 J r1 J r 2
Cylinder
r1
r2
Diffusion area of 2πrl
is not fixed
j
C
2 lD ln(r )
Diffusivity of carbon
For a given experiment, j can be determined by chemical analysis, and then
we can determine D from the slope of the plot of C versus ln(r).
At 1000oC
Carbon content
Concentration profile of carbon in
the wall of a hollow steel cylinder
under conditions of steady-state
diffusion.
j
C
2 lD ln(r )
Diffusion coefficient of carbon in
iron varying with its concentration
at 1000℃
J = C v C ( B F ) CB (
)
x
ln a
C
)C D I
x
x
ln a
ln C
BRT
DI
x
x
∂ lna
ln X
ln
∴ D I = BRT
BRT
)
BRT (1
∂ lnC
ln X
ln X
BRT (
Steady State Diffusion
P1
Glass
C1
1
H 2 [ H ]metal foil
2
P2
metal foil
C2
Sievert’s law
kequilibrium
* Diffusion of hydrogen gas through
the metal foil is the rate-limiting
step.
*Equilibrium concentrations
(C1 and C2) on both sides,
determined by the local
partial pressures.
*Steady State achieved.
(area is fixed)
constant
[ H ]metal foil
PH 2
C1 H metal 1 k PH 2 1
C2 H metal 2 k PH 2 2
P(H2-1) and P(H2-2) are partial pressures of
hydrogen on sides 1 and 2, respectively
C1 C2
k
C
( PH 2 1 PH 2 2 )
x
Dk
C
J x D
( PH 2 1 PH 2 2 )
x
Consider an iron tank that contains H2 gas at a pressure of Po at a
temperature of To. If the pressure outside the tank is zero, after what
time the pressure in the tank has decreased to half of its original value?
Rate of loss of mass from tank (Δm/Δt)
= mass diffusion flux through walls (JxA)
Assuming H2 is an ideal gas
dm MV dP
dt
RT dt
Dk
Jx
P
(M: molecular weight of H2 and
V: volume of tank)
(PV=nRT=(m/M)RT)
dm
(A: Area of the tank)
Jx A
dt
DkA
MV dP
P
RT dt
P dP
t DkART
P0 P 0 MV dt (k :equilibrium constant)
H2 Permeation through Metal Foil
H2
Adsorption
H
Diffusion
J reaction kP
H
Chemical Reaction
n
k: Reaction rate constant
P: Partial pressure of H2
P
J adsorption
2 MkBT
α: Sticking coefficient
M : Molecular weight
H2 Permeation through Metal Foil
1.Adsorption
2.Chemical Reaction
3.Diffusion
4.Chemical Reaction
5.Desorption
J
R1
R2
C
x
V
C
I
J
x
R
D
J D
R3
R5
R4
Process with steps in series
the slowest one controls the
whole reaction, and the rest
reach their equilibrium states.
Steady State
(1) Diffusion controlling process
CIIeq
C
x
Since ΔC is fixed by chemical reactions
(assuming constant D), J is inversely
proportional to x.
Time
J D
(2) Reaction controlling process
J is independent of x, and
CIeq
x
CIIS
D is inaccessible.
Time
(3) Mixed controlling process
CIIeq
CIeq (reversible)
0 (irreversible)
Time
CIeq
(4) Extreme Cases
CIIeq
Reaction control
Mixed control
CIeq Diffusion control
x
Steady State
J: constant (fixed A)
J
C
C
x ( D
)0
t
x
x
x
C
C
(D
)
t x
x
2C C 2 D
D 2 ( )
x
x C
(a) steady state and D≠D(C)
D is not necessary
to be constant.
C
2C
C
0 D 2 0
= constant
t
x
x
(b) steady state and D=D(C) e.g ., D 0 and
a=
0
a>
0
0
a<
C
D=Do+ aC
X
C
2C C 2
D 2 ( ) a 0
t
x
x
2C
(1)a 0 D 2 0 convex ()
x
2C
(2)a 0 D 2 0 straight line (-)
x
2C
(3)a 0 D 2 0 concave ()
x
D
a
C
(C) Steady State, D=D(x)
C
c o n s ta n t
x
Jdx D dC
if D a x
J D
Jdx axdC
dx
dC
a
x
J
a
C
ln x
J
J 1
C
a x
x
J 1
2C
a x2
x 2
2C
0
*a 0 D
2
x
2C
0
*a 0 D
2
x
Steady state
J : constant
C
( )x 0
t
(1) D ≠ D(C)
J D
C
C
D
x
x
I.C. C(0, 0) = C0
C(x, 0) = Ci
B.C. C(0,t)=C0
J x D C
J (0 x ) D (C0 Ci )
Ci C o
Jx
D
(2) D = D(C) = aC
J aC
x
0
J
dx
a
C
x
Ci
Co
C dC
Jx
1 2
(Ci C02 )
a
2
Transient State --Thin-film solution
(Infinite Sink)
A quantity of solute, S, is plated as a thin film on one end of a long rod of solute-free
material, then a similar solute-free rod is welded to the plated end.
Annealed for time (t) Determine concentration profile of the solute C(x,t).
C*
2δ
Assuming
D ≠ D(x)
(Constant diffusivity)
C
2C
Fick's 2nd Law :
=D 2
t
x
I.C. C(x,0)= 0
C(x,0)= C*
B.C. C(∞,t)=0
C(-∞,t)=0
|x|>δ
|x|≤δ
Conservation of mass
C ( x, t )dx S ' ( 2 C * )
S’= g/cm2 (Surface Concentration)
C*= g/cm3
A
x2
General Solution : C ( x, t )
exp(
)
4 Dt
t
'
2
S
x
exp(
) (Note:
Particular Solution : C ( x, t )
4 Dt
2 Dt
C (0, t )
(A: constant)
Dt 2 )
S'
2 Dt
x2
C ( x, t ) C (0, t ) exp(
)
4 Dt
Taking the natural logarithm of both sides yields
C ( x, t )
x2
ln(
)
C (0, t )
4 Dt
Thus a graph of ln( C ( x, t ) C (0, t ) ) against x 2 should yield a straight line
with a slope of 1 4 Dt .
Transient State --Thin-film solution
(Infinite Sink)
S'
x2
exp(
)
C ( x, t )
4 Dt
2 Dt
2.0
Dt=0
0.025
C/S’
1.5
Time
0.05
0.075
1.0
0.25
1.0
0.5
3.0
0
-3
-2
-1
0
X (Distance)
1
2
3
ln[C(x,t)/C(0,t)]
Determine D
-1/4Dt
x2
if ln(
C ( x, t )
) vs. x 2 is not a linear relation, D is a function of concentration.
C (0, t )
If it is linear, D is independent of concentration.
S'
x2
exp(
)
C ( x, t )
4 Dt
2 Dt
C
(1)
|x 0 0 Impermeable Boundary
x
1
S'
(2)C (0, t )
C (0, t )
2 Dt
t
x2
(3) when
1 x 2 Dt
4 Dt
S'
C (0, t )
exp(1)
this plane is determined.
C ( x, t )
e
2 Dt
x
t this plane ( x 2 Dt ) moves away from x 0.
C
|x 0 0 Impermeable Boundary
x
Examples:
(1)
Setter
(2)
Al2O3 coating
Thin-film Solution:
S'
x2
C ( x, t )
exp(
)
4 Dt
2 Dt
1.0
(1) Constant Area
0.8
(1)
0
-4
-3
-2
C/S’
2 Dt
123
(3)
-5
S'
312
(2)
0.2
C ( x, t )dx S ' ( 2 C * )
(2) C (0, t )
0.6
0.4
-1
0
x
1
2
3
4
Concentration-distance curves for an instantaneous plane source.
5
Thin Film Solution Gaussian Concentration Distribution
S'
x2
exp(
)
C ( x, t )
4
Dt
2 Dt
x2
exp( 4Dt )dx 2 Dt
C ( x, t )dx S '
(4) Reflection at a boundary (e.g. metal coated with oxides)
2x
C
|x 0 0 (Impermeable Boundary)
x
* Reflection and superposition are mathematically sound
* Linear sum of the two solutions is itself a solution
*C ( x , t )
x2
exp(
)
4Dt
Dt
S
'
Superposition and Reflection
∂C
| x=0 = 0
∂x
Fictitious
source
x2
S'
C ( x, t) =
exp()
4D
t
2
πDt
+
S'
x2
C ( x, t) =
exp()
4D t
2 πD t
x>0
x<0
ll
C( x, t) =
S'
x2
exp() x>0
4Dt
πDt
0.6
C( x, t) =
0.5
S'
x2
exp() x>0
4Dt
πDt
0.4
x2
S'
C( x, t) =
exp()
4Dt
2 πDt
S'
x2
C( x, t) =
exp()
4Dt
2 πDt
x>0
0.3
x<0
0.2
0.1
0
-4
-3
-2
-1
0
1
2
3
4
Superposition and Reflection
-1
0
+1
-1
-1
0
+
0
C( x, t) = C( x, t) C( x, t) =
C ( x, t) =
S'
( x + 1) 2
exp()
4D
t
πDt
C( x, t) =
S'
( x - 1) 2
exp()
4Dt
πDt
+1
+1
S'
( x +1)2
( x - 1)2
[exp() + exp()]
4D
t
4
Dt
πDt
Thin Film Solution
(a)
C
X=0
(b)
X
∂C
∂x
J =-D
(c)
C
x
Accumulation
∂C
∂2C
=D 2
∂t
∂x
2
∂C
∂x2
* Please derive (b) and (c)
Depletion
Thin Film Solution
(a)
C
X
X=0
(b)
-J
∂C
∂x
J=0
+J
J =-D
C
x
Maximum
Flux
(c)
∂C
∂2C
=D 2
∂t
∂x
2
∂C
∂x2
Maximum
Depletion
Zero
Accumulation
Thin Film Solution
x2
)
C ( x , t ) C (0, t ) ex p (
4Dt
x2
C
2x
)
C (0, t ) ex p (
4Dt
4Dt
x
C
x 0
0
J 0
x
C
x 0
J 0
0
x
C
X
X=0
∂C
∂x
+
J
-
+J
-J
Flux to the left is negative
Flux to the right is positive
Leak Test
S'
x2
exp(
)
C ( x, t )
4 Dt
2 Dt
2.0
Dt=0
0.025
1.5
C/S’
0.05
0.075
1.0
0.25
0.5
-3
1.0
B.C. C(-∞,t)≠0
-2
3.0
-1
0
X (Distance)
B.C. C(∞,t)≠0
1
2
3
Leak Test
(Superposition and Reflection)
C*
C**
C*
+
C**
Concentration at a given point will be
higher than that of “Infinite Case”
because of reflection.
Leak Test
Sample Dimension
1.2
1
0.8
0.6
0.4
0.2
-∞
-6
-4
-l
0
-2
0
0
2
l
4
∞
6
The above analyses are only good for a thin film in the middle of
an “Infinite Bar”. If it is not infinite, the diffusion will be reflected
back into the specimen when it reaches the end of the bar, and
concentration in that region will be higher than the above solution.
Q: How long is long enough to be considered infinite?
Leak Test
Arbitrarily taking 0.1% as a sufficiently insignificant concentration
2
x
exp(
)dx
(
,
)
C
x
t
dx
l
4 Dt
3
1
0.1% l
0
2 ' Dt
2
S
x
exp(
)dx
0 C ( x, t )dx 0
4 Dt
2 Dt
x
dx
, du
Let u
2 Dt
2 Dt
x u
l
xl u
2 Dt
S'
S'
3
10
S'
l
2 Dt
0
exp(u 2 )du
exp(u 2 )du
l
)
erfc(
l
2
Dt
)
1 erf (
1
2 Dt
l 4.6 Dt
l 4.6 Dt
(Check the Table of Error Function)
The bar is considered to be long enough
to use a thin-film solution with 99.9%
accuracy.
Error Function
erf ( z )
erf ( ) 1
2
z
0
exp( u 2 ) du
erf (0) 0
erf ( z ) erf ( z )
Complementary
Error Function
1 erf ( z ) erfc ( z )
Small z erf ( z )
2
2
n0
z
exp( u 2 ) du
( 1) n z 2 n 1
(2 n 1) n !
exp( z 2 ) 1
1
Large z ( z 3) erfc ( z )
( 3 ........)
z 2z
2
d ( erf ( z ))
exp( z 2 )
dz
4z
d 2 ( erf ( z ))
2
exp(
z
)
2
dz
Error Function
2
erf ( z )
z
0
exp(u 2 )du
Table 1-1. The Error Function
erf(z)
z
erf(z)
0
0
0.85
0.7707
0.025
0.0282
0.90
0.7969
0.05
0.0564
0.95
0.8209
0.10
0.1125
1.0
0.8427
0.15
0.1680
1.1
0.8802
0.20
0.2227
1.2
0.9103
0.25
0.2763
1.3
0.9340
0.30
0.3286
1.4
0.9523
0.35
0.3794
1.5
0.9661
0.40
0.4284
1.6
0.9763
0.45
0.4755
1.7
0.9838
0.50
0.5205
1.8
0.9891
0.55
0.5633
1.9
0.9928
0.60
0.6039
2.0
0.9953
0.65
0.6420
2.2
0.9981
0.70
0.6778
2.4
0.9993
0.75
0.7112
2.6
0.9998
0.80
0.7421
2.8
0.9999
1.0
erf(z)
z
0.5
0
0
1.0
z
2.0
3.0
ierfc( x) erfc(u )du
x
1
exp( x 2 ) x[1 erf ( x)]
x
Solution for a pair of Semi-infinite Solids
C’
C
i
0
0
x
I .C. C ( x,0) 0 x 0
C ( x,0) C ' x 0
B.C. C (, t ) C '
C (, t ) 0
Superposition
(1) No interaction from adjacent slabs
(2) Superposition of the distributions
from the individual slabs since the
diffusion equation is linear and additive.
n
x i )
C'
C ( x, t )
exp(
4 Dt
2 Dt i 1
0 n
2
C'
C ( x, t )
2 Dt
Δα
c e(x2)2/4Dt
2 Dt
C
*
C(x,t)
0
0
α2
X
0
x i
exp(
4 Dt
2
)d
Δα
c e ( x 2 ) 2 /4 D t
2 Dt
*
C
C(x,t)
0
0
X
α2
x i )
C '
exp(
Ci ( x, t )
4 Dt
2 Dt
Note : S C '
2
Sum the solution of all thin slabs
2
n
x i
C'
C ( x, t )
exp(
)
Dt
4
2 Dt i 1
0 n
C ( x, t )
Substituting u
C
2
'
Dt
0
exp(
x i
2
4 Dt
x
, d 2 Dtdu
2 Dt
) d
x
2 Dt
u
0u
C'
C ( x, t )
x
2 Dt
exp(u 2 )du
reverse limits of integration and split integral
0
x
2 Dt
0
C ( x, t ) (
)
C'
exp(u 2 )du
By definition of error function
2
erf ( z )
erf ( ) 1
z
0
exp( u 2 ) du
erf (0) 0
erf ( z ) erf ( z )
C( x, t )
C'
C'
x
2 Dt
0
0
[ exp(u 2 )du
[
2
2
erf (
x
)]
2 Dt
C'
x
)]
[1 erf (
2
2 Dt
exp(u 2 )du]
1
(2)
C
C0
0.5erf ( x )
2 Dt
0.5
0.5erf ( x ) 0.5
2 Dt
0
0.5
0
X
Note:
If the concentration is fixed (C=C*),
the term of x / 2 Dt is then also fixed.
This means that the penetration distance
is a function of the square root of
the diffusion time. For example, if a
diffusion penetration of 0.1mm
develops in one hour, it will take
4 hours to develop a penetration
of 0.2 mm.
C 1
x
[1 erf (
)]
C0 2
2 Dt
C
1
x
0.5 erf (
(1) x 0
)
C0
2
2 Dt
x
1
C
0.5 erf (
x0
)
C0
2
2 Dt
x
C
1
0.921
(2)
C0
2 Dt
which varies with time as x 2 Dt
(all compositions except C
(3) x 0
Co
0.5).
C 1
(implicit B.C.)
C0 2
C D
C
|x 0 0
(-:to the left)
2 t
x
(5) Total mass crosses the plane at x 0
(4) J x 0 D
t
M
Dt
Jdt C0
0
A
(A:area)
Error Function Short-time Solution
C/Co
1.0
0.5
Time
0
x/l
The above analyses are only good for an “Infinite Slab”.
If it is not infinite, the diffusion will be reflected
back into the specimen when it reaches the end of the bar, and
concentration in that region will be higher than the above solution.
Q: How long is long enough to be considered infinite?
Leak Test
Arbitrarily taking 0.1% as a sufficiently insignificant concentration
Co
0
l
1
C0 [1 erf (
(
,
)
C
x
t
dx
l
2
2
l
0.1%
1
(
,
)
C
x
t
dx
0
0 2 C0[1 erf ( 2
x
)]dx
Dt
103
x
)]dx
Dt
Using B.C. to solve the problem
1
t im
0.5
x
)
2 Dt
C (, t ) C ' A B
time
0
C ( x, t ) A B erf (
e
C
Co
t∞
C (, t ) 0 A B A B
0
X
C ( x, t )
C'
2
C'
x
[1 erf (
)]
2
2 Dt
Examples
(1) if C(0, t) = 0
C ( x, t ) C 'erf (
x
)
2 Dt
(2) if C(0, t) = C”=A, C(∞,t)=C’
C ( x, t ) C "[1 erf (
x
)],
2 Dt
C C"
x
),
erf (
C ' C "
2 Dt
x0
x0
(next page)
C (0, t ) C "
C'
B.C.
C''
C (, t ) C '
C (, t ) 0
x0
C ( x, t ) A Berf (
0
X<0
0
X>0
x
)
2 Dt
C (0, t ) C " A
C (, t ) 0 A B C "
x
)]
C ( x, t ) C "[1 erf (
2 Dt
x0
C (0, t ) C " A
C ( , t ) C ' A B C ' B C ' C "
x
)
C ( x, t ) C " (C ' C " )erf (
2 Dt
C'
C
2
"
if
C'
x
)
x 0, C ( x, t ) (1 erf (
2
2 Dt
'
C'
x
' C
)
x 0, C ( x, t ) (C )erf (
2
2
2 Dt
C'
x
(1 erf (
)
2
2 Dt
C'
C'/2
0
X<0
0
X>0
Error Function & its Derivatives
(2)
(1)
(1)
(3)
(4)
(1) erf( x )
d(erf( x ))
≡ (-)Fl u x
dx
d 2 (er f( x ))
≡ Accumulation
(3)
dx2
d 3 (erf( x ))
(4)
dx 3
Note: (2) is the thin film solution
(2)
Semi-infinite Solution
x
C ( x, t ) A B erf (
)
2 Dt
B
x2
C
exp(
)
x
4Dt
Dt
B 0
C
0 J< 0
x
C
X=0
∂C
∂x
J
+ +
- -
X
Flux to the left
is negative
C
X=0
J
∂C
∂x
+ +
- -
X
Flux to the right
is positive
Doping of Semiconductors
n-type
Donors: Sb, As, P
p-type
Acceptors: B, Al, Ga
Diffusion from a Limited Source (thin film)
P
p-type Si
2
C (0, t ) constant
1.8
C
1.6
o
0
1.4
t <t <t <t
1
1.2
2
3
C ( x , t ) dx S 0 constant
S0
x2
C ( x, t )
exp(
)
4 Dt
Dt
4
:Gaussian function
1
Example: p-n junction
0.8
C0.6'
t
0.4
t Time t
X
1
2
0.2
C’0
-0.2
0
0.5
xj
1
t
3
1.5
C’= Background Concentration
4
C C ' ( p n or n p )
x
2
2.5
x
3
2
x
S
0
C'
exp( j )
4 Dt
Dt
x j junction distance
Diffusion from a Constant Source
P
p-type Si
p-type Si
P
C (0, t ) C0
Co
C’
xj
t1 t2
x
C ( x, t ) C0erfc(
)
2 Dt
t1< t2< t3< t4
Dt
0
M A C ( x, t )dx 2 AC0
t3
t4
: the amount of dopant entering the base
x
Example: p-n junction
C C ' ( p n or n p)
C’= Background Concentration
xj
); x j : junction distance
C C0 e rfc(
2 Dt
'
Example
1.0
C/Co
I .C. C ( x, 0) f ( x ') a x b
B.C. C (, t ) 0, C (-, t ) 0
f(x’)
C ( x, t ) lim
'
0.5
x 0
n 1
f ( x' )x '
( x x' )2
exp(
)
4 Dt
2 Dt
f ( x ') : initial distribution of concentration
Time
0
x’
a
b
x
when x ' 0
f ( x' )
( x x' )2
C ( x, t )
exp(
)dx '
4 Dt
2 Dt
f ( x' )
( x x' )2
( )(
exp(
))dx'
4 Dt
2 Dt
a b
a
b
f ( x' )
( x x' )2
exp(
)dx '
a
4 Dt
2 Dt
when f ( x' ) Ci
b
C ( x, t )
b
a
( x x' )2 '
exp(
)dx
4 Dt
2 Dt
Ci
x x'
Let u
dx' 2 Dtdu
2 Dt
xa
x b
'
'
x a u
, x b u
2 Dt
2 Dt
xb
2 Dt
x a
2 Dt
C( x, t )
Ci
Ci
2 Dt
xb
2 Dt
x a
2 Dt
exp(u2 )(2 Dt )du
exp(u2 )du
x b
xa
[erf (
) erf (
)]
2
2 Dt
2 Dt
Ci
Ci
xa
x b
) erf (
)]
[erf (
2
2 Dt
2 Dt
Ci
B.C.
C (, t ) C (, t ) 0
0
a
b
0
a
b
Ci
Ci
x - a ) - C i er f ( x - b )
erf (
2
2
2 Dt
2 Dt
C
x - a ) - erf ( x - b )]
i [ erf (
2
2 Dt
2 Dt
C0
Time
0
a
b
x
Co
x - b )]
C ( x, t ) [1 erf (
2
2 Dt
C0
+
0
a
b
x
Co
x
-a
)]
C ( x, t ) [1 erf (
2
2 Dt
ll
Co
x
-a
x
-b
C ( x, t ) Co - [erf (
) - erf (
)]
2
2 Dt
2 Dt
C0
Time
0
C0
-
a
b
x
b
x
C0
Time
0
C ( x, t ) Co -
a
Co
x - a ) - erf ( x - b )]
[erf (
2
2 Dt
2 Dt
B.C. C(-a,t)=C(a,t)=Co
-a
(a)
0
a
C ( x , t ) C o e r fc (
-a
(b)
0
+
ax)
2 Dt
a
a-x
C ( x, t ) Co erfc(
)
2 Dt
-a
0
a
a- x
a x
(a) (b) C( x, t ) Co [erfc(
) erfc(
)]
2 Dt
2 Dt
Semi-infinite
(1)
f(x')
x = -∞
x=∞
C ( x, 0) f ( x ' )
C (0, t ) 0
f ( x' ) f ( x' ) odd function
f(-x')
'
f (x )
f ( x ' )
( x x' )2 '
( x x' )2 '
C ( x, t )
exp(
)dx
exp(
)dx
0
4
Dt
4
Dt
2 Dt
2 Dt
0
(2)
f(-x')
f(x')
C ( x, 0) f ( x ' )
C
(0, t ) 0
x
f ( x' ) f ( x' ) even function
-∞
0
'
f (x )
f ( x' )
( x x' )2 '
( x x' )2 '
C ( x, t )
exp(
)dx
exp(
)dx
0
4
Dt
4
Dt
2 Dt
2 Dt
0
∞
I.C.
C(x,0)=Co
C(x,0)=0
Co
a
≣
0
Co
y
-a
-y
Fictitious
Source
a
B.C.
C(0,t)=0
0≤x≤a
x>a, x<0
Equivalent I.C.
C(x,0)=Co 0≤x≤a
C(x,0)=-Co –a≤x≤0
C(x,0)=0,x>a, x<-a
-Co
Co
x
x-a
[erf (
) - erf (
)]
2
2 Dt
2 Dt
C
-x
-x - a
(- o )[erf (
) - erf (
)]
2
2 Dt
2 Dt
C
x
x-a
xa
o [2erf (
) - erf (
) - erf (
)]
2
2 Dt
2 Dt
2 Dt
C ( x, t )
Example
I .C.
Ci
0
x
C ( x, 0) Ci
x0
C ( x, 0) 0
x0
B.C.
C (0, t ) 0
C (, t ) Ci
Ci
Equivalent I .C.
C ( x,0) Ci
Fictitious
Source
-Ci
C ( x,0) Ci
x0
x0
f ( x' )
( x x' )2
C ( x, t )
exp(
)dx '
4 Dt
2 Dt
'
f (x )
( x x' )2
exp(
)dx '
0
4 Dt
2 Dt
f ( x ' ) Ci , x ' 0; f ( x ' ) Ci x ' 0
0
C ( x, t ) Ci erf (
x
)
2 Dt
( x 0)
Example
Ci
-l
-h
0
h
l
-l
-h
0
h
l 2l-h 2l 2l+h 3l
I .C.
C ( x, 0) Ci
h x h
C ( x, 0) 0
x h, x h
-l -h
h
-l -h
h
l
B.C.
C ( x, t )
0 x l , x l
x
Equivalent
I .C.
C ( x,0) Ci 2nl h x 2nl h , n
f ( x' )
( x x' )2
C ( x, t )
exp(
)dx '
4 Dt
2 Dt
2 nl h
Ci
( x x' )2
exp(
)dx '
2 nl h
4 Dt
2 Dt
Ci
x (2nl h)
x (2nl h)
[erf (
) erf (
)]
2
2 Dt
2 Dt
l
Example
B.C.
C ( x, t )
0 x l , x l
x
Equivalent
-l -h
h
-l -h
h
l
-l -h
h
l
-l -h
h
l
l
I .C.
C ( x,0) Ci 2nl h x 2nl h , n
Example
C ( x, 0) Ci
Ci
C (0, t ) Cs
Define C Cs
( x, 0) Ci Cs i
(0, t ) 0
( x, t ) i erf (
Cs
0
x
)
2 Dt
C Cs (Ci Cs )erf (
Time
x
)
2 Dt
C Cs
x
erf (
)
Ci Cs
2 Dt
x
Example: Carburization
A piece of AISI 1020 steel is heated to 1255K and subjected to a
carburizing atmosphere such that
2CO CO2 C( s )
(1)
is in equilibrium with 1 wt%C in solution at the surface
1.0
C
(wt%)
Time
0.2
Cs is fixed by eq. (1) =1 wt%C
C2=0.2 wt%C
Dc 2 x107 cm2 s 1 at 1255K
C Cs
x
)
erfc(
C2 Cs
2 Dt
When t = 3600 s
C=0.35 wt%C at x = 5x10-2 cm
Fe-C Phase Diagram
Separation of Variables
1.0
C/Co 0.5
0
Time
-5 -4 -3 -2 -1 0 1 2 3 4 5
x/h
Series Solutions
Small system + long time
Real solution to all systems without assumptions of “Infinite System”
Assuming the solution can be represented by
C ( x , t ) ( x )T ( t )
2C
C
D
assu m in g
2
t
x
2
dT
DT
x 2
dt
( x )T ' ( t ) D T "
D D (C )
Divide both sides by C ( x, t )
dT
"
dt DT
T
T
T'
"
DT
T'
: function only of time
DT
"
: function only of distance
Since they vary independently, both sides must be equal to a constant,
designated as -λ2 where λis a real number
1 dT
2 D
T dt
T T0 exp( 2 Dt )
where T0 is a constant, -λ2 is chosen because one deals with the system
in which any inhomogeneities disappear as time passes, i.e., T approaches
zero as time increases.
The equation in
x is
d 2
2 0
dx
the solution to this equation is of the form
( x) A' sin( x) B cos( x)
where A’ and B’ are functions of λ
C ( x, t ) ( x )T (t )
T0 exp( 2 Dt )( A' sin x B cos x )
( A sin x B cos x ) exp( 2 Dt )
But if this solution holds for any real value ofλ, then a sum of solutions
with different values ofλ is also a solution. Thus in its most general form
the product solution will be infinite series of the form
C ( x, t ) [( An sin n x Bn cos n x) exp(n 2 Dt )]
n 1
Example :”Diffusion out of a Slab”
Co
I .C . C ( x, 0) Co 0 x h
B.C. C ( x, t ) 0 x 0 and x h
C
(
x
,
t
)
C (0, t ) 0 Bn 0
[( An sin n x Bn cos n x ) exp( n 2 Dt )]
n 1
C ( h, t ) 0 the argument of sin n x is equal to zero
Time
0
h
n n where n is a positive integer
h
C ( x, t ) ( An sin n x )(exp( n 2 Dt ))
n 1
C0 C ( x, 0) An sin n x (0 x h)
n 1
An sin(
n 1
n
x) An ?
h
M ultiplying both sides by s in( p x
) and integrate x over the rang e of 0 x h to determine An
h
h
h
p x
n x
p x
C
dx
A
sin
(
)
sin
(
)
s
in
(
) dx
0
n
0
0
h
h
h
n 1
n p
n 1
n p
n 1
h
An s in (
0
h
An sin (
0
n x
p x
) sin(
) dx 0
h
h
n x
p x
h
) sin(
) d x An
2
h
h
0
2C0
2C0
2 h
n x
h
n x
n x
An C0 sin(
)dx
( ) cos(
)
cos(
)
0
h
h
h
n
h 0 n
h h
h
2C0
2C
n 0
n h
[cos(
) cos(
)] 0 [1 cos(n )]
n
h
h
n
n : even An 0
4C0
4C0
n : odd 2 j 1 An
n
(2 j 1)
The solution is C ( x, t )
j 0,1, 2......
(2 j 1) x
(2 j 1) 2
1
sin(
)
exp(
(
) Dt )
h
h
j 0 (2 j 1)
4C0
Note: Each successive term is smaller than the preceding one, and the percentage
decreases between terms and increases exponentially with time. Thus after a short
time has elapsed, the infinite series can be satisfactorily represented by only a few
terms. To determine the error, we compare the ratio of the maximum values of the
first and second terms (R)
8 2 Dt
R 3exp(
2
)
h
R 100 when h 4.75 Dt
h2
t
(4.75) 2 D
The error in using the first term to represent C(x,t) is less than 1% at all points.
Degassing of Metals
It is difficult to measure the concentration of gas at various depths, and what is
experimentally determined is the quantity of gas which has been given off or the
quantity remaining in the metal. Therefore, the average concentration ( C ) is used.
1 h
C ( x, t )dx
0
h
8C0
1
(2 j 1) 2
exp(
(
) Dt )
2
2
h
j 0 (2 j 1)
C
When C (t ) 0.8C0 the first term is a good approximation to the solution or when t is
sufficiently large
(1st term is not
a good approximation)
8
C
t
2 exp( )
C0
h2
2 : relaxation time
D
Large slow process
is not linear
n C
Co
D
1
t
Fourier Series
Example: Initial concentration is a sum of sine series
∞
C o = A n sin (λ n x )
n=1
n=1
∞
nπ
x)
= A n sin (
h
n=1
y
n=2
n=3
n=3
Y
x
h
Co
y
An sin(n x)
n 1
Y
w hen x h
0
h
x
Fourier Series
C
B.C.
C ( x, t ) 2
x 0, , 2 ........
-π
π
0
2π
3π
s i n ( 2 j 1) x
0
2 j1
8
C o ( x, 0) 2, C1( x, 0) 2
s in ( x )
C ( x, 0) 2
8
8
s in (3 x )
3
8
8
8
C 19 ( x , 0 ) 2
s in ( x )
s i n ( 3 x ) ......
s in (3 9 x )
3
3 9
C 2 (x, 0) 2
8
s in ( x )
C
-- j=1
-- j=2
-- j=3
-- j=11
x
Example:
Co
Time
I .C .
C ( x, 0) 0 0 x h
C ( x, 0) C0 x 0, x h
B.C .
C ( x, t ) C0
C C0
x 0, h
1
(2 j 1) x
(2 j 1) 2
sin(
)
exp(
(
) Dt )
(2
j
1)
h
h
j 0
4C0
1 h
C ( x, t ) dx
h 0
8
1
(2 j 1) 2
C C0 [1 2
exp(
(
) Dt )]
j 0 (2 j 1) 2
h
C
0
h
Example:
I .C.
C ( x,0) Ci
Ci
Cs
-L
C ( x,0) 0
x L, x L
B.C.
C
(0, t ) 0
x
C ( L, t ) Cs
Time
0
L x L
L
C Cs 4 (1)n
(2n 1) x
(2n 1)2 2 Dt
cos(
) exp(
)
4
2
L
Ci Cs n0 (2n 1)
L2
1 L
C ( x, t )dx
0
L
C Cs
8
1
(2n 1)2 2 Dt
exp[
]
2
2
2
4
Ci Cs n0 (2n 1)
L
C
8
t
exp(
) (only the first term is taken)
2
4L2
where 2
D
Phase Transformation in Pb-Sn Alloy System
Solidification of an off-eutectic Alloy -- Coring
Transverse section
Example : Homogenization of Alloys
Coring: Diffusion of most alloying element in the solid state is too slow to maintain a
solid if uniform concentration is in equilibrium with the liquid during solidification.
Dendrite
As-cast
completely homogenized
last to solidify
Co
time
short time
first to solidify
0
L
0
L
I .C.: C ( x, 0) f ( x)
C
B.C.:
(0, t ) 0
x
C
( L, t ) 0
x
x
C ( x, t ) C0 An cos
An
n x
Dt
exp(n 2 2 2 )
L
L
2 L
n x
(
)
cos
f
x
dx
L 0
L
(Ref: Crank’s Book p.63)
Numerical Approximations
Error Function Series
C
0 at x 0 and x l.
x
0
l
Sequence of mirror reflection to provide no-flow boundary conditions
Co
0
h
I.C.
C ( x, 0) Co
0 xh
C ( x, 0) 0
h xl
B.C.
C
0
x
l
∵∂C/∂x|x=0 =0
x 0, x l
∂C/∂x =0
Co
( x x ') 2
C ( x, t )
exp(
) dx '
h
4 Dt
2 Dt
h
-h 0
-l
h
l
Co
∵∂C/∂x|x=l =0
∂C/∂x|x=l =0
∂C/∂x =0
Co
-h 0
-l
h
l
2l
3l
2l h
Co
Co
( x x ') 2
( x x ') 2
exp(
)dx '
exp(
)dx '
C ( x, t )
2
h
l
h
4 Dt
4 Dt
2 Dt
2 Dt
h
C
|x 0 0,
x
C ( x, t )
0
C
| x l 0. ........
x
2 nl h
2 nl h
Co
( x x ') 2
exp(
) dx '
4 Dt
2 Dt
x x'
u
2 Dt
C o
x 2 nl h
x 2 nl h
C ( x, t )
( [ erf (
) erf (
)])
2 0
2 Dt
2 Dt
0 xl
Error Function (3 terms)
1.0
C/Co
Time
0.5
0
x/l
2 Dt
h 1
l 3
x 1
x 1
3
3
l
l
[erf (
) erf (
)]
2 Dt
2 Dt
l
l
1
1
1
x
x
2
2
C ( x , t; )
l 3
1
3 ) erf ( l
3 )]
[erf ( l
2
C0
2 Dt
2 Dt
l
l
1
1
x
x
2
2
3 ) erf ( l
3 )]
[erf ( l
2 Dt
2 Dt
l
l
Note: When
l increases, more error function terms would be needed
because error functions spread indefinitely and therefore do not provide
support of the no-flow boundary conditions.
Fourier Series (15 terms)
1.2
h 1
l 3
1
C/C0
0.8
2(Dt)1/2/l=0
Time
0.6
0.4
0.75
0.2
-0.2
0
0.2
0.4
∞
0.5
0.1 0.25
0.025
0
0
1
0.6
0.8
1
Dimensionless Distance (x/l)
C ( x, t ) 1 2 1
n
n x
n 2 Dt 2
sin( ) cos(
) exp[( ) 2 (
) ]
C0
3 n 1 n
3
l
2
l
Note: At short times (small 2 Dt l ), more Fourier terms would be needed to
eliminate spurious ripples and negative concentrations in the solution.
Short Time Solution
2 Dt
0.025
l
1.2
h 1
l 3
1
0.8
C/C0
0.6
Error Function
0.4
Fourier Series
(15 term)
2(Dt)1/2/l=0.025
0.2
0
-0.2
0
0.2
0.4
0.6
Dimensionless Distance (x/l)
0.8
1
Method of Laplace Transform
Laplace transform to the diffusion equation is to remove the time variable leaving an
ordinary differential equation, the solution of which yields the transform of the
concentration as a function of the space variables (x,y,z). (Solve p.d.e. by making it an o.d.e.)
Definition
pt
F ( p) e f (t )dt
0
F(p) is called the Laplace transform of the original function f(t), and denoted by L(f(t))
pt
L( f ) F ( p) e
0
This operation is called Laplace Transform.
1
f (t )dt
f (t ) L ( F )
is called inverse transform or inverse of F(p)
f (t )
L( f )
1
1
1
t
t
2
t
n
e
p
2
p
2!
p
n!
p
f (t )
3
n 1
at
cos(w t)
s in ( w t )
cosh(at)
s in h ( a t )
L( f )
1
p
p
p 2
w
p 2
p
p 2
a
p 2 a
a
w
2
w
2
a
2
2
L( f ' ) pL( f ) f (0)
L( f ) p 2 L( f ) pf (0) f (0)
Example: Semi-infinite System
I.C.
C ( x, 0) 0 x 0
B.C.
C (0, t ) C0
Co
Time
0
x
(Note: diffusion in a semi-infinite medium, x>0 when the boundary is
kept at a fixed concentration. e.g. doping problem)
C
2C
D 2
t
x
multiplying both sides by e-pt and integrating with respect to time
from 0 to ∞, one obtains
pt
e
0
2C
1 pt C
dt e
dt 0
2
0
D
t
x
Assuming the orders of differentiation and integration can be interchanged
pt
e
0
2C
2 pt
2 C
dt 2 0 Ce dt 2
2
x
x
x
Note : C L (C ) F ( p ) C ( x, t )e pt dt
0
C
d t p L (C ) C (0 ) p C 0
0
t
I.C.
N o te : L ( f ' ) p L ( f ) f ( 0 )
e pt
2C
p
C 0
2
x
D
C A1 e
p
D x
B .C . : C ( 0 , t ) C 0
A2 e
p
L (C 0 )
D x
C0
C
p
C ( , t ) 0 A2 0
C
C
pD0
C A1 e
0 A1 0
p
p
C 0 p D x
C
e
p
F r o m T a b le L 1 ( C ) C
x
C C 0 e r fc (
)
2 Dt
Example
I .C .
C ( x , 0) 0 l x l
B .C .
C ( x, t ) C0 x l, x l
C (0, t )
0
x
2C
p
C 0 0 xl
x 2
D
C
0
at x 0
x
C
at x l , x l
C 0
p
C 0 cosh( p D x )
C
p cosh( p D l )
Co
Time
-L
0
L
ex ex
N ote: cosh( x ) =
2
Note: please check Crank’s Book pages 22-24 to have the solution
C C0
4C 0
(2 n 1) 2 2 D t
(2 n 1) x
( 1) n
exp(
)
cos(
)
2
4
2
l
l
n 0 (2 n 1)
Example: Constant Flux at the surface
I .C . : C ( x , 0) C 0
x0
B .C . : C ( , t ) C 0
J (0, t ) A D C (0, t ) x
C
C
2C
D
t
x 2
Co
0
x
C0
2C p
C
D
x 2 D
C k1e
p
x
D
k2e
p
x
D
k3
C ( , t ) C 0 k1 0
C ( x, p ) k2e
L ( D
p
x
D
k3
C (0, t )
C (0, p )
A
) D
L ( A)
p
x
x
A
C (0, p )
Dp
x
p
C ( x, p )
k2e
D
x
p
x
D
p
A
C (0, p )
k2
x
D
Dp
k2
A
1
D 2p
3
2
To get k3, go back to partial differential equations
C0
2 C ( x, p ) p
(
,
)
C
x
p
x 2
D
D
p
p
x
x
C
p
p
k 2e D [k2e D k3 ] 0
D
D
D
C
C
p
k3 0 k3 0
D
D
p
A
C ( x, p )
D
q
p
1
2
p
3
e
p
x
D
2
C0
p
C ( x, p ) k 2 e
(*)
(Ref:Crank Book No.9)
D
1
2
qx
2
x
x
Dt 4 Dt
1 e
) 2
(
)
L (
e
xerfc
pq
2 Dt
qx
1
2
e
D e
3
pq
p2
p
x
D
A
D
C
A 1
L 1 [ eq .(*)]
L (Crank No.9) L 1 ( o )
D
p
multiplied by
p
x
D
k3
A Dt 12 4xDt
x
C ( x, t ) 2( ) e
xerfc(
) C0
D
2 Dt
Check C ( x, 0) C0
2
C (0, t )
A
x
x2
1
C ( x, t ) A Dt 2 4 Dt
2x
x
x
(
) erfc(
) x (erfc(
))
2( ) e
x
4 Dt
D
x
2 Dt
2 Dt
C (0, t )
C (0, t )
A
A D
x
x
D
D
2 A Dt 12
C (0, t )
( ) C0
D
Solutions for Variable D
C
C
D C
2C
)
(D
D 2
t x
x
x x
x
D
x makes this equation inhomogeneous, especially when D=D(C) or D(T) or D(t) or D(x).
The key in solving the above p.d.e. is to simplify the equation with x and t to x or t function.
Boltzman-Matano Analysis (D=D(C))
x
t
C C
1 x C
3
t t
2 t 2
and
Therefore
C C
1 C
x x
t
1 x C D C
3
(
)
2 t 2 x t
C
1
(D
)
t
C
C
(D
)
2
Example: Infinite System
Co
Original interface
I.C. C(x,0)=Co x<0η= -∞
C(x,0)=0 x>0η= ∞
* x=0 is not determined yet
If D≠D(C), C=Co/2 which determines x=0 for an
infinite system. However, if D=D(C) the above
condition is no longer valid, the x=0 must be
determined by
Time Matano
interface
0
Co
X=0
Co
C’
Tangent=(dC/dx)C’
C
)
0 2
0
1 C' x
dC C '
dC C '
C C '
dC D
|0 D
| D t
|0
x 0
x
d
2 0 t
d( )
t
dC C '
1 C'
xdC Dt
|0
dx
2 0
X=0
xdC 0
which expresses the equality of the two shaded areas.
Equal area
Matano
interface
C0
C'
C'
dC d ( D
Error-Function Solution
1.0
Constant Diffusivity
C/Co
Loss
C0
0
xdC 0
0.5
Gain
0
0
x/l
Error-Function Solution
1.0
Constant Diffusivity
C/Co
Loss
C0
0
xdC 0
0.5
Gain
0
0
x/l
D increases with decreasing concentration
For an infinite system
dC
0 when C 0 or C Co
dx
dC C0
|0 0
dx
Matano
interface
Co
Therefore
1 C0
dC C0
|0
xdC Dt
0
dx
2
dC C0
|0 0
dx
C’
0
X=0
Tangent=(dC/dx)C’
C0
xdC 0 which is an additional boundary
0
condition and determines the
location of Matano interface.
x=0 plane (Matano interface) determined by
C0
0
xdC 0
C'
1 dx
D (C ) ( )C ' xdC
0
2t dC
'
1 C'
dC C '
Note : xdC Dt
|0
2 0
dx
t =0
C1
C2
Original interface
t =t
C1
Matano interface
Areas equal
D1
C2
D2
x=0
C1
C*
D(C ' )
Slope=dC/dx at C*
C**
C2
1 dx
( )C ' xdC
0
2t dC
C'
0
∂C
∂C
|C * >
|C **
∂x
∂x
D iffu s iv ity : D C * < D C **
S lo p e :
A rea :
C
C1
*
C
x d C = C x d C
2
**
t=0
C’
3
2
1
C'
-1 dx
D(C') = (
)C' xdC
0
2t dC
D1 (C') > D 2 (C') > D 3 (C')
x xM
t
C'
1 dx
D(C ') ( )C ' ( x xM )dC
0
2t dC
Matano Interface
Loss
CL
[CL C ( x)]dx [C ( x) 0]dx
xM
Integrated by parts ( udv uv vdu )
[CL C ( x)] x |xM
CM
xM x’
x
Gain
x’: Original interface
xM: Matano interface
CL
CM
C’(x)
0
xM
xM
[C ( x) 0] x |
0
CM
xdC
xdC
B.C.: C (, t ) CL ; C (, t ) 0
(CL CM ) xM
CL
CM
0
CM
xdC (CM 0) xM
xdC 0
(CL 0) xM
CL
CM
(CL 0) xM (
xdC
CL
CM
0
CM
xdC
xdC 0
CM
0
xdC ) 0
If the coordinate of the Matano interface, xM, is selected as
the origin of the x-axis, i.e., xM=0, then
CM
CL
xdC
0
0
CL
CM
xdC 0
CL
xdC dC 0
0
Although the location of xM=0 is not known a priori, it may be found
from concentration-distance data by balancing the area denoted
Loss against the area of Gain.
Loss
CL
CM
C’(x)
0
xM x’
x
Gain
Hardening of Steels
THROUGH HARDENING In order to harden steel, the iron mix must contain a certain amount of carbon.
Carbon dissolves in molten iron. In through hardening steel, there is a high level of carbon added to the
iron mix. When the component is heat treated, it becomes hard all the way through from the surface to
the core, hence the term “through hardened”. Through-hardened steel components are relatively brittle
and can fracture under impact or shock load.
The Moving Boundary Problem
*Diffusion controlling process along with reaction at phase boundary
General Aspects
* CⅠ* and CII* : equilibrium concentrations in
phases I and II.
C0
CI *
II
CⅠ
2CⅠ
DⅠ 2
*xS
t
x
CⅡ
2CⅡ
xS
DⅡ 2
t
x
I
CII*
Cs
S
x
* Diffusion controlling process
*
CⅠ* kCⅡ
k : partition ratio between phases
* DⅠ(
CⅠ
C
dS
) xS DⅡ( Ⅱ ) xS (CⅡ* CⅠ* )
dt
x
x
Kinetic Issue dS
dt ?
Temperature
Decarburization
T1
γ
α
α+γ
Concentration
Free Energy
Cα Cγ
α
= or
γ
C
C
C
C
a =a
α
α+γ
γ
Concentration
Microstructure
Cα Cγ
Cγ
Cα
α
To have net diffusion flux
between and , the
thickness of + has to
vanish.
γ
α+γ
α
γ
S
x
Carburization: -Fe -Fe
Decarburization: -Fe -Fe
T
Ci1
Ci2
910°C
γ
T*
723°C
α+γ
γ+Fe3C
α
Cα*
0.025
C (wt%)
Cγ* 0.83
Ci1
Decarburization
α
Cγ*
γ
Cα*
Cs
T=T*
S
Cs
γ
Ci2
x
Cα*
S
α
μc,
ac
S
x
μ Cα =μ Cγ at x=S
where α and γ coexist
T=T*
Cγ*
Carburization
Decarburization
forming phase
at the interface
Carburization
forming phase
at the interface
x
CO
C*I
II
C*II
CS
0
I
dS
S
Mass Conservation
(JII - JI )A dt = (CII* - CI* )A dS
∂CII
∂CI
JII - JI = -DII (
) x=S - (-DI
) x=S
∂x
∂x
A: area for diffusion: constant
Fe-C Phase Diagram
Carburization
Cs
C *
C *
T=T*
Co
0
S
x
dS ?
dt
known parameters:
Cγ* , Cα* , CS , Co , DγC and DαC
Example:
Carburization
*Chemical activity of carbon at surface can be set up by
k
C CO2
2CO
k[CO]2
ac
; or
[CO2 ]
k
2H 2 C
CH 4
ac
k[CH 4 ]
PH 2 2
*Rate of advance of boundary controlled by diffusion of carbon in Fe. Therefore
xS
C* kC*
(if C* C* at x S reaction controlling process )
*
C C* at x S
C C* at x S
* Semi-infinite solid
*
DC D(C ) DC DC
*
V 0
mass flux requiring no density correction
2
C
C C
Fick’s 2nd law
D
xS
t
x 2
2
C
C
DC
0 xS
2
t
x
I .C. : C ( x, 0) C0
Cs
C *
C *
B.C.: in phase
Co
C C*
at x S
C C0
at x
in
x
S
0
phase
C C*
at x S
C Cs
at x 0
C ( s, t ) C* kC*
J
J
Solute
entering
Solute
receiving
ds
(k : Partition Ratio)
At x S
( J J )dt (C* C* )dS
C
C
dS
) x s (C* C* )
x
x
dt
Note : ( J J )dt A (C* C* )dS A
DC (
) x s DC (
In phase
C A B erfc(
x
2 D t
C
), x C C0 A
In phase
C A ' B ' erf (
x
2 DC t
C ( x, t ) C0 B erfc(
C ( x, t ) Cs B ' erf (
), x S C C* ; x 0 C Cs A '
x
2 D t
C
x
2 D t
C
Cs
)
C *
)
C
At boundary x S C C*
C Cs B ' erf (
*
S
2 D t
C
)
C and CS are constants ( diffusion controlling process)
S
2 D t
C
is a constant too
S
2 D t
C
(constant)
S 2 DC t where is a constant
C ( S , t ) C* Cs B ' erf (
Co
*
therefore
*
S
2 DC t
) Cs B ' erf ( )
0
S
x
Similarly
C ( S , t ) C* C0 B erfc(
Replace
S
2 D t
C
) C0 B erfc(
DC
DC
1
C ( S , t ) C0 B erfc( 2 ) C*
D (
C
C
x
) xs
DC B ' 2
2 DC t
x2
exp(
) |x s (1)
C
4 D t
C
DC B 2
x2
) xs
exp(
) |x s (2)
D (
C
C
4 D t
x
2 D t
C
Eq.(1) (2) (C* C* )(
S
)
t
S 2 DC t
DC
dS
dt
t
C* C*
B ' e
(Note: it is not a constant)
2
2
Be
2 DC t
2 D t
C
)
Cs B ' erf ( ) C*
Use them to estimate B’ and B,
and then to solve β
1
C0 Berfc ( 2 ) C*
Cs C*
C C
*
*
2
e erf ( )
C* C0
1
2
1
2 e erfc ( 2 )
solve by trial and error
DC
dS
then we get S 2 D t and
dt
t
C
1
2
e erfc( )
0
β
Note: the only unknown parameter in the above equation is β
(C* , C* , Cs , C0 , DC and DC are known parameters )
Example: Decarburization
0.4% Carbon Alloy Steel
T=800oC
Cs=0.01% (equilibrium by CO and CO2)
t = 30 min S-Fe?
Answer: Co=0.4%, Cs=0.01%
C*=0.24%, C*=0.02% (Obtained from Fe-C Phase Diagram)
DC 3x108 ; DC 2x106 cm 2 /sec
DC
C
D
66.6
(C C )
*
Cs C*
*
2
e erf ( )
C* C0
1
2
2
1
e erfc( 2 )
(0.01 0.02) (0.24 0.4)
f ( )
f ( 1/2 )
0.01 (0.24 0.4)
0.22
f ( )
f ( 1/ 2 )
by trial and error 0.144 ( see Figure)
(0.02 0.24)
S 2 DC t 0.0173 cm
Fe-C
Phase Diagram
Formation of a single-phase layer from an initial two-phase mixture
In the two-phase region, the average composition, Co, is assumed to be uniform,
which requires, in effect, that the grain size is small and that second-phase dispersion
is uniform.
Cs
CI
723<T<910℃
*
723<T<910℃
CI*
Cs
Co
S
De-carburization
S
Carburization
T<723℃
CI*
Cs
Co
Co
α+Fe
Fe33cC
T>723℃
CI*
+Fe3C
Cs
S
De-carburization
Co
S
De-carburization
CII ( S , t ) CII*
CII
dS
) x s (CII* C0 )
x
dt
Cs CII* B2erf ( )
DII (
C C0
*
II
B2 exp( 2 )
Cs CII*
eliminate B2
( *
) exp( 2 )erf ( )
CII C0
1
Formation of a single-phase layer from an initial two-phase mixture
In the two-phase region, the average composition, Co, is assumed to be uniform,
which requires, in effect, that the grain size is small and that second-phase dispersion
is uniform.
Cs
CI
723<T<910℃
*
723<T<910℃
CI*
Cs
Co
S
De-carburization
S
Carburization
T<723℃
CI*
Cs
Co
Co
α+Fe
Fe33cC
T>723℃
CI*
+Fe3C
Cs
S
De-carburization
Co
S
De-carburization
CII ( S , t ) CII*
CII
dS
) x s (CII* C0 )
x
dt
Cs CII* B2erf ( )
DII (
C C0
*
II
B2 exp( 2 )
Cs CII*
eliminate B2
( *
) exp( 2 )erf ( )
CII C0
1
Carburization Process
723<T<910oC
CS
CII*
Time
Co
α+γ
γ
S
Steady state without Interfacial resistance (immobile boundary)
C1
a1
I
C2 *
II
I
II
a*
C1*
a*
a2
C2
Δx
Δx
Concentration profile
Chem. activity profile
a* k2C2*
C1 C1*
J1 D1
x
C2* C2
J 2 D2
x
a* k1C1*
C1* kC2*
k2 k1k
k1 and k2 are activity coefficient
Steady State J1 = J2
No interfacial resistance
C1* C2* k k (
Note:
C1*
k (const )
C2*
D1C1 D2C2
)
D2 kD1
D1 D2 C1* C1
D1 D2 C2* C2
k is a partition coefficient
DI>>DII
I
II
CII*
Diffusion
Control in
Phase II
I
CI
CI
DI<<DII
I
II
CII*
CI*
CII*
CI*
CI*
Diffusion
Control in
Phase I
II
I
Reaction @
interface of
Phase I &
diffusion
control
in Phase II
II
CI
CII*
CI*
Mixed
diffusion
control
Infinite composite system without Interfacial Resistance
C0
C*1
C
1
I .C.: C ( x, 0) C0
x0
C ( x, 0) 0
x0
B.C.: C (0, t ) C1*
Chemical
activity
C2*2
C
0
C (0, t ) C 2* x 0
No accumulation at the interface
C 2*
k at x 0
C1*
D1
x=0
C1
C
D2 2
x1
x2
C1 A1 B1erf (
C0
C 1 (0, t )
D 1
1 k( 2 ) 2
D1
kC 0
C 2 (0, t )
D 1
1 k( 2 ) 2
D1
*
at x 0
x
)
2 D1t
C2 A2 B2 erf (
*
x 0
x
)
2 D2t
(immobile boundary)
x0
x0
C1
C0
D 1
x
{1 k ( 2 ) 2 erf (
)}
D2 12
D
2
D
t
1
1
1 k( )
D1
C2
x
kC0
erfc(
)
D2 1 2
D
t
2
2
1 k( )
D1
(both of them are fixed)
(k=1, D2/D1=1C1*=C2*=Co/2)
Composite system with Interfacial Resistance (immobile boundary)
C0
Chemical
activity
Contact Resistance at x=0
C1
h(C1 C2 ) x 0
x
C
C
D1 1 D2 2 h(C1 C2 )
x
x
D1
0.5Co
t
C2
C1
t
0
0
The concentrations on either side of the interface
are no longer constant, but each approaches the
equilibrium value of 1/2C0 relatively slowly. (if D1=D2)
C1
C0
D 1
x
x
{1 ( 2 ) 2 {erf (
) exp(h1 x h12 D1t ) erfc[
h1 D1t ]}}
D2 12
D
2 D1t
2 D1t
1
1 ( )
D1
C2
x
x
C0
h2 D2t )}
{erfc(
) exp(h2 x h22 D2t ) erfc(
D2 12
2 D2t
2 D2t
1 ( )
D1
h1
D 1
h
{1 ( 1 ) 2 }
D1
D2
D 1
h
h2
{1 ( 2 ) 2 }
D2
D1
h: Chemical reaction rate constant at interface
(if D2=D1, C1(0,∞)=C2(0,∞)=Co/2)