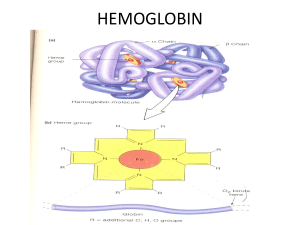
IRON METABOLISM AND IRON DEFICIENCY ANEMIA Awal Mir M.Phil MLS, PhD Scholar IRON Iron is an essential element required for energy production, oxygen use, and cellular proliferation Each cell in the body needs iron, not too much and not too little Iron is ionized compound, overload lead to iron toxicity resulting organ damage Conversely, if too little iron is available, the synthesis of physiologically active iron compounds is limited, and critical metabolic processes are inhibited 3 IRON CONTAINING COMPOUNDS 4 SOURCES OF IRON Sources of Iron Non-Dietary iron Source (85%) Dietary iron (10-15 %) Plant/non-heme source (Ferric form) • • • • • Apple Pear Spinach Date Bean Animal/heme source (Ferrous form) • • • • Red meat Beef or chicken Liver Fish egg Erythropoiesis Recycling Globin Heme Hemoglobin Normal RBC destruction 5 DAILY IRON REQUIREMENTS 6 IRON CYCLE 7 IRON DISTRIBUTION Most body iron is present in haemoglobin in circulating red blood cells (1.7-2.4 g) The macrophages of the reticuloendothelial system store iron released from haemoglobin as ferritin and hemosiderin (0.3-1.5 g) Myoglobin is also contain 0.15 g iron Transporting iron (transferrin): 0.003-0.004 g Heme enzymes: 0.02-0.02 g Approximately 1 to 2 mg iron losses each day in urine, faeces, skin and nails and in menstruating females as blood and 1 to 2 8 mg iron is absorb from diets in duodenum. DIETARY IRON ABSORPTION Dietary ingredients ingest about 15 mg iron of which only 10% will be absorbed HCL in stomach detach iron from food particles. Iron is absorbed in upper part of the duodenum in slight acid medium Iron is absorbed in ferrous form. Ferric form is first converted into ferrous form by action of ferrireductase enzyme present on brush border of enterocyte Transportation of iron from GI tract to bone marrow via transferrin 9 DIETARY IRON ABSORPTION 10 DIETARY IRON ABSORPTION 11 IRON TRANSPORTATION AND STORAGE Transferrin is iron containing transporter protein composed of appo-transferrin and two iron molecules Iron is absorbed in ferrous form and transported in ferric form so it is first converted into ferric form by the action of oxygenase enzyme before to bind with appo-transfferin 1 gram of transferrin binds 1.4 mg of iron (total iron binding capacity) Iron is utilize in bone marrow for the developing normoblast for use of hemoglobin synthesis or its is store in macrophages of reticuloendothelial system in the form of ferritin or hemosiderin 12 IRON UTILIZATION IN B.M 13 IRON STORAGE Iron is stored mainly in the liver in reticuloendothelial system as Hemosiderin Ferritin Hemosiderin is the major long term storage form of iron ; release slowly, Ferritin is the primary storage form of soluble iron ;release readily at time of need. 14 PROTEINS INVOLVED IN IRON HOMEOSTASIS 15 IRON METABOLISM DISORDERS IDA = Iron deficiency anemia, ACD = Anemia of chronic disease, SA = Sidroblastic anemia. 16 IRON DEFICIENCY ANEMIA It is a clinical condition characterized by low level of hemoglobin due to depletion of iron stores in the body lead to mild to severe anemia 17 IRON DEFICIENCY ANEMIA IDA is the most common nutritional deficiency anemia in the world. Globally about 20% of women, 90% of pregnant women and 3% of men present with iron deficiency anemia. On the bases of severity IDA is divided into 3 categories. Mild anemia (With hemoglobin level 9-12 g/dl) Moderate anemia (With hemoglobin level 6-9g/dl) Severe anemia (With hemoglobin level <6g/dl) 18 ETIOLOGY OF IDA Increased Iron demand/Utilization Pregnancy Infancy Adolescence Blood Loss/iron loss (1 ml blood loss = 0.5 mg iron loss) Gastrointestinal Tract (hemorrhoid, hook worm, gastritis) Menstrual Blood Loss Nose bleeding (Epistaxis) Urinary Blood Loss (hematuria and hemoglobinemia and hemosidrinuria) Mal-absorption Tropical Sprue Gastrectomy Chronic atrophic gastritis Poor iron diet intake 19 PATHOPHYSIOLOGY OF IDA Iron deficiency anaemia develops in three stages Iron depletion: Iron stores decreases (low ferritin) but no anemias and erythrocyte morphology is normal. Elevated RDW. Iron deficient erythropoiesis: There is insufficient iron to insert into the protoporphyrin ring to form heme. Serum iron is also depleted but no anaemia and hypochromia. Erythrocytes may became slightly microcytic Iron deficiency anaemia: A long-standing negative iron flow eventually leads to the last stage of iron deficiency. All laboratory tests for iron status become markedly abnormal. Classic microcytosis and hypochromia 20 CLINICAL FEATURES Generalize sign and symptoms • • • • • Pallor Fatigability Dizziness Headache Shortness of breath Specific sign and symptoms • • • • Koilonychias (Spoon shaped nails) Glossitis (Inflammation of the tongue) Angular cheilosis (Ulcerations corner of the mouth) Pica syndrome (Appetite for non food substances such as clay) Geophagia (clay eating tendency), Phagophagia (ice eating tendency), Amylophagia (Starch eating tendency) 21 CLINICAL FEATURES 22 LABORATORY DIAGNOSIS Complete blood count and peripheral blood smear examination Hb: decrease MCV: decrease TLC: normal/increase MCH: decrease Platelets: normal MCHC: decrease RBC count: decrease RDW: markedly increase HCT: decrease Retic count: decrease Peripheral blood film reveals anisopoikilocytosis with microcytosis, hypochromia, eliptocytosis (pencil cells) and few tear drop and target cells. WBCs are normal. Platelets are high (reactive thrombocytosis) on film examined. 23 LABORATORY DIAGNOSIS 24 LABORATORY DIAGNOSIS Serum Iron Profile Serum total iron Decreased Serum ferritin Decreased TIBC(total iron binding capacity) Increased Tansferrin saturation % Decreased Bone marrow examination (Not required for IDA diagnosis) Hypercellur marrow with micronormoblastic erythropoiesis. Myelopoiesis is active with all stages of maturation. Megakaryopoiesis is normal. Iron stain shows no stainable iron seen. 25 LABORATORY DIAGNOSIS 26 Thank You Any Question.? 27 SIDROBLASTIC ANEMIA Awal Mir M.Phil MLS OUTLINE INTRODUCTION CLASSIFICATION OF SA PATHOPHYSIOLOGY OF SA RESULTS AND DISCUSSION CONCLUSION AND RECOMMENDATION REFERENCES 3 INTRODUCTION Sideroblastic anemias are a heterogeneous group of disorders characterized by defect in heme biosynthesis lead to variable number of microcytic and hypochromic red cells in the peripheral blood and ringed sideroblasts in the bone marrow. 4 INTRODUCTION Numerically they are an uncommon group of anemias. The term sideroblasts refers to the nucleated red cells which contain iron granules in their cytoplasm Under physiological conditions iron which is delivered to the proerythroblasts and the basophilic erythroblasts is utilized for the synthesis of hemoglobin In SA it is not incorporated into mitochondria where heme synthases take place and deposited in erythroblast These iron granules can be stained with a special stain called Prussian blue stain 5 CLASSIFICATION OF SIDROBLASTIC ANEMIA Classification of Sidroblastic Anemia Acquired Hereditary X linked recessive Drugs & chemicals • Isoniazid, • Pyrazinamide, • Chronic lead toxicity • Chloramphenicol • Alcohol Autosomal recessive Immunological disorders • Polyarteritis nodosa • Rheumatoid arthritis • Myxedema Secondary Primary Malignant Pyridoxin Pyridoxin disorders responsive unresponsive • Lymphomas • Plasma cell Nutritionals dyscrasias Metabolic • Malabsorption disorders • MPN • Folate • Uremia 6 deficiency HEREDITARY SA The most common form of hereditary SA is sex linked and due to an abnormal delta-aminolevulinic acid synthase 2 (ALAS2) enzyme More than 22 different mutations have been identified The mutations are located in exons 5–11 with most being single-base mutations affecting the site at which the enzyme binds the cofactor pyridoxal 5′ phosphate This lead to ineffective erythropoiesis and bone marrow erythroid hyperplasia Erythroid hyperplasia results in increased iron absorption in the gut Iron overload can be significant and can lead to complications such as cardiac failure and diabetes 7 ACQUIRED SA Lead and alcohol are the most common causes of this form of SA Lead poisoning (plumbism) has been recognized for centuries Once ingested, lead passes through the blood to the bone marrow where it accumulates in the mitochondria of erythroblasts and inhibits cellular enzymes involved in heme synthesis The heme enzymes most sensitive to lead inhibition are delta-aminolevulinic acid synthetase and ferrocheletase. 8 ACQUIRED SA Heme synthases is disturbed at the conversion of delta-ALA to porphobilinogen and incorporation of iron into protoporphyrin to form heme. lead poisoning consistently shortens the erythrocyte life span and decrease heme synthesis lead to anemia. Alcohol is also interfere with some enzymes of hemoglobin synthesis including inhibition of the synthesis of pyridoxal phosphate and of activity of uroporphyrinogen decarboxylase and ferrochelatase but enhancement of activity of delta-ALAS.9 PATHOPHYSIOLOGY In SA there is disturbances of the enzymes regulating heme synthesis Several types of biochemical disorders exist including abnormalities of glycine, ALA & porphyrin metabolism Due to heme enzyme deficiency mitochondria eventually rupture as they become iron overloaded This non ferritin iron appears as blue deposits circling the nucleus formed ring sideroblasts Anemia is resulting due ineffective erythropoiesis, decrease heme syntheses & the mean erythrocyte span varies between 40 days 10 CLINICAL FEATURES Patients with hereditary SA or RARS, generally show primary signs and symptoms of anemia • Pallor • Fatigability • Dizziness • Headache • Shortness of breath Acquired SA symptoms are according to underlying disease Signs associated with iron overload including hepatomegaly, splenomegaly, and diabetes and latter stages cardiac function can be affected 11 LABORATORY DIAGNOSIS Complete blood count and peripheral blood smear examination Hb: decrease MCV: decrease TLC: normal MCH: decrease Platelets: normal MCHC: decrease RBC count: decrease RDW: increase HCT: decrease Retic count: decrease Peripheral blood film reveals anisopoikilocytosis with dimorphic blood picture (microcytes, normocytes) and few tear drop and target cells. WBCs are normal. Platelets are normal on film examined. 12 LABORATORY DIAGNOSIS Serum Iron Profile Serum total iron Increased Serum ferritin Increased TIBC(total iron binding capacity) Decreased Tansferrin saturation % Increased Bone marrow examination (Not required for IDA diagnosis) Hypercellur marrow with erythropoiesis with ring sidroblast. Myelopoiesis is active with all stages of maturation. Megakaryopoiesis is normal. Iron stain is Positive. 13 LABORATORY DIAGNOSIS 14 Thank You Any Question.? 15 FOLATE /VITAMIN B12 METABOLISMS AND MEGALOBLASTIC ANEMIA Awal Mir M.Phil MLSc, PhD Scholar OUTLINE INTRODUCTION TO MACROCYTIC ANEMIA FOLIC ACID METABOLISM COBLAMIN (VITAMIN B12) METABOLISM MEGALOBLASTIC ANEMIA ETIOLOGY OF MEGALOBLASTIC ANEMIA CLINICAL FEATURE AND LAB DIAGNOSIS 3 INTRODUCTION TO MACROCYTIC ANEMIA In macrocytic anemia the red cells are abnormally large in size (MCV >100 fL) On the bases of morphological characteristic of erythroid precursor in the bone marrow macrocytic anemias are classified into megaloblastic and nonmegaloblastic (normoblastic) anemia Megaloblastic erythroid precursors in the bone marrow lead to oval macrocytes in peripheral blood while round macrocytosis is noted in non megaloblastic anemia However both type of macrocytosis is frequently a sign of a disease process that can result in significant morbidity if left untreated 4 CAUSES OF MACROCYTIC ANEMIA 5 VITAMIN B12 METABOLISM Sources of cobalamin or vit. B12 Cobalamin is produced in nature only by microorganisms, and humans receive cobalamin solely from the diet Human obtain it by eating food of animal origin or by eating bacterially contaminated foods Animal protein is the major dietary cobalamin sources Fish, muscle meats, milk products, and egg yolk is richest sources of cobalamin (1 to 10 μg/100 g of weight) 6 VITAMIN B12 METABOLISM Absorption of cobalamin Cobalamin in food is usually in coenzyme form adenosylcobalamin and methylcobalamin, nonspecifically bound to proteins A normal diet contains a large excess of B12 compared with daily needs Cobalamin is released from protein‐binding in food by digestion at the low pH in stomach 7 VITAMIN B12 METABOLISM Absorption of cobalamin It combined with the glycoprotein intrinsic factor (IF), which is synthesized by the gastric parietal cells Cobalamin+IF complex binds in the ileum to a specific surface receptor for IF, cubilin. This binds to a second protein, amnionless, which directs endocytosis of the cubilin IF–B12 complex in the ileal cell so that B12 is absorbed and IF destroyed 8 VITAMIN B12 METABOLISM Transportation of cobalamin Vitamin B12 is absorbed into portal blood where it becomes attached to the plasma‐binding protein transcobalamin (TC) and it is further three types. TC-1 or haptocorrin is an α1 globulin which carries from 70-90% of circulating vitamin B12. It is primarily a storage protein and its absence doesn’t lead to clinical signs of B12 deficiency. 9 VITAMIN B12 METABOLISM Transportation of cobalamin TC-2 is a β-globulin which carries vitamin B12 organ to organ and in & out of cells. Congenital deficiency of TC-2 leads to severe megaloblastic anemia TC-3 is a similar to TC-1, binds only a small quantity of circulating B12. 10 VITAMIN B12 METABOLISM 11 VITAMIN B12 METABOLISM 12 VITAMIN B12 METABOLISM 13 CAUSES OF COBALAMIN DEFICIENCY 14 FOLATE METABOLISM Sources of Folate Plants sources: Leafy vegetables(spinach, lettuce, broccoli) Fruits (bananas, melons, lemons) Beans Yeast Mushrooms Animals sources: animal meats Microorganisms 15 FOLATE METABOLISM Absorption and transportation Folate in food is in the conjugated polyglutamate form. It is deconjugated in the intestine to a monoglutamate prior to absorption. Absorption can take place throughout the small intestine but is especially significant in the proximal jejunum. Once taken up by the intestinal epithelial cell, the folate is reduced to methyl THF, the primary circulating form of THF in the blood. Methyl THF is distributed throughout the body via the blood and attaches to cells by means of specific receptors called cell surface folate receptor. 16 FOLATE METABOLISM 17 CAUSES OF FOLATE DEFICIENCY 18 MEGALOBLASTIC ANEMIA Megaloblastic anemia is a clinical condition characterized by defective nuclear maturation caused by impaired DNA synthesis lead to megaloblasts in the bone marrow and oval macrocytosis in the peripheral blood resulting pancytopenia or bicytopenia. 19 ETILOGY OF MEGALOBLASTIC ANEMIA Vitamin B12 deficiency or defect in vitamin B12 metabolism Folic acid deficiency or defect in folic acid metabolism Both vitamin B12 and folic acid deficiency Therapy with antifolate drugs. (e.g. methotrexate) Therapy with drugs interfering with synthesis of DNA. (e.g. cytosine arabinoside) 20 NUTRITIONAL ASPECTS 21 PERNICIOUS ANEMIA This is caused by autoimmune attack on the gastric mucosa leading to atrophy of the stomach. The wall of the stomach becomes thin, with a plasma cell and lymphoid infiltrate of inner lining. Intestinal metaplasia may occur There is secretion of IF is absent Helicobater pylori infection may initiate an autoimmune gastritis which presents in younger subjects as iron deficiency and in the elderly as PA 22 PATHOPHYSIOLOGY DNA is formed by polymerization of the four deoxyribonucleoside triphosphates. Folate deficiency is thought to cause megaloblastic anemia by inhibiting thymidylate synthesis, a rate‐limiting step in DNA synthesis in which deoxythymidine monophosphate (dTMP) is synthesized. This reaction needs 5,10‐methylene THF polyglutamate as coenzyme. The role of B12 in DNA synthesis is indirect. 23 PATHOPHYSIOLOGY B12 is needed in the conversion of methyl THF, which enters marrow and other cells from plasma, to THF. Defective nuclear maturation and megaloblastic morphology of cells is caused by decreased thymidine triphosphate synthesis from uridine monophosphate (UMP). This deficiency interferes with nuclear maturation, During replication when thymidine triphosphate is not present in adequate amounts deoxyuridine triphosphate incorporates into the DNA. This mis-incorporation causes delayed maturation of the nuclear chromatin, fragmentation of the nucleus and immature cell destruction. 24 PATHOPHYSIOLOGY 25 CLINICAL FEATURES Patients present mildly jaundiced (pallor) due to dyserythropoiesis in bone marrow. Glossitis (a beefy‐red sore tongue) Angular cheilosis Weight loss due to epithelial abnormality Purpura as a result of thrombocytopenia Vitamin B12 neuropathy (sub acute combined degeneration of the cord) 26 CLINICAL FEATURES Pallor (Megaloblastic anemia) Angular cheilosis 27 CLINICAL FEATURES Glossitis (a beefy‐red sore tongue) Neural tube defect (spina bifida) 28 LABORATORY DIAGNOSIS Complete blood count and peripheral blood smear examination Hb: decreased MCV: Increased TLC: Decreased/normal MCH: Normal Platelets: Decreased MCHC: Normal RBC count: Decreased RDW: increased HCT: Decreased Retic count: decreased Peripheral blood film shows anisopoikilocytosis with marked oval macrocytosis, tear drop, target cells and nucleated RBCs. Leukopenia with hypersegmented neutrophils and giant band cells. Thrombocytopenia with giant platelets on film examined. 29 LABORATORY DIAGNOSIS 30 LABORATORY DIAGNOSIS Biochemical investigations Serum Vitamin B12 Decreased Serum folate level Decreased Bilirubin (unconjugated) Increased Serum LDH Increased Bone marrow examination (Indicated for pancytopenia) Hypercellur marrow with megaloblastic dysplastic erythropioesis. Myelopoiesis shows dysplastic changes with giant myelocyte and metamyelocytes. Megakaryopoiesis is normal. 31 Iron stain is Positive. Thank You Any Question.? 32 HEMOGLOBIN DISORDERS AND THALASSEMIA SYNDROME Awal Mir M.Phil MLS NORMAL HUMAN HEMOGLOBIN Embryonic Hemoglobin (early embryogenesis hemoglobin) • Hb Gower 1 (ζ2,ε2) • Hb portland (ζ2, γ2) • Hb Gower 2 (α2, ε2) Fetal Hemoglobin (Intra-uterine life hemoglobin) • Hb F (α2,γ2) Adult Hemoglobin (after birth or adult hemoglobin) • Hb A (α2,β2) 98% • Hb A2 (α2,δ2) 1.5- 3.2% • Hb F (α2,γ2) <1% 3 NORMAL HEMOGLOBIN SYNTHASES The genes for the globin chains occur in two clusters: ε, γ, δ, β on chromosome 11 and ζ , α on chromosome 16 All the globin genes have three exons and two introns with locus control region (LCR) LCR is a genetic regulatory element, situated upstream of the globin genes clusters, that controls genetic activity by opening up the chromatin to allow transcription factors (BCL11A) to bind Globin chain synthases take place by the process of transcription, mRNA processing and translation 4 NORMAL HEMOGLOBIN SYNTHASES 5 HEMOGLOBIN DISORDERS Hemoglobin disorders or hemoglobinopathies are hereditary genetic disorder of hemoglobin and affect approximately 7% of the world population It is arising due to mutations in one or more globin chain genes resulting hemoglobin structural defect (qualitative disorder) or quantitative hemoglobin defect or coexistence Qualitative hemoglobinopathy is characterized by changes in amino acid sequence of globin chain such as hemoglobin S In Hb S there is substitution of valine for glutamic acid in position 6 in the β chain (146 amino acids) Quantitative hemoglobinopathy is characterized by absence or decrease of one or more globin chain synthesis but hemoglobin is 6 normal in structure such as thalassemia syndrome HEMOGLOBINOPATHY Hemoglobinopathies Quantitative hemoglobinopathies (decrease hemoglobin quantity) Thalassemia syndrome α Thal • Silent carrier • α thal trait/minor • Hb H disease • Hb Bart disease γ Thal δ Thal β Thal • β thal trait/minor • β thal intermedia • β thal major Qualitative hemoglobinopathies (hemoglobin structural defect) • • • • • • Hb S (Sickle cell anemia) Hb D Hb C Hb E Hb O Hb G and etc. Coexistence hemoglobinopathies • Beta trait with HbS trait • Beta trait with HbE trait • Beta trait with HbD trait 7 and etc. THALASSEMIA SYNDROME The thalassemia syndromes are a heterogeneous group of inherited anemias characterized by defects in the synthesis of one or more of the globin chain subunits of the hemoglobin tetramer Thalassemia comes from a Greek word “Thalas” meaning the sea and “emia” for blood The disease was first diagnosed in people from the Italian and Greek coasts and nearby Mediterranean sea It is a well-established fact that thalassemia is widely distributed all over the world In 1925, two American pediatricians, Thomas Cooley and Lee first described thalassemia as a disease characterized by severe anemia, splenomegaly and bone deformities 8 THALASSEMIA SYNDROME Thalassemia syndrome is classified according to globin chain deficiency α-globin chains are absent or reduced in patients with α-thalassemia, β-globin chains in patients with β-thalassemia, δ-globin in patients δβ-thalassemia and γ globin in patients γβ-thalassemia α-thalassemia and β-thalassemia is major category and more common thalassemia in the world while the rare forms include the γ-, δ- and εγδβ- thalassaemias α-thalassemia is rare in Pakistan and β-thalassemia is most common about 5 to 7 percent population of Pakistan is carrier for beta thalassemia gene 9 CLASSIFICATION OF THALASSEMIA On the bases of type of globin chain deficiency α thalassemia β thalassemia δβ thalassemia γβ thalassemia HbS thalassemia HbE thalassemia HbD thalassemia On the bases of clinical severity of disease β thalassemia α thalassemia β thalassemia minor Silent α thalassemia β thalassemia intermedia α thalassemia trait β thalassemia major HbH disease Hydrops fetalis 10 CLASSIFICATION OF THALASSEMIA Molecular bases classification of Thalassemia 11 ALPHA THALASSEMIA Alpha thalassemia syndrome is a hereditary genetic disorder characterized by absence or reduced α-chains synthesis due to gene deletions or inactivation The clinical severity is related to the number of the four α‐globin genes missing or inactive Loss of all four genes completely suppresses α‐chain synthesis α-chains is essential in fetal as well as in adult hemoglobin this is incompatible with life and leads to death in utero In α-thalassemia there is deficiency of α-chain and excess of γ-chain in fetus while excess of β-chain in after birth Excess of free γ-chain become tetramer known as Hb Bart and tetramer of beta chain known as Hb H 12 α THALASSEMIA GENETIC POLYMORPHISM Clinical nomenclature Genetic defect Genotype Silent carrier One gene deletion αα/-α α Thalassemia trait Two gene deletion -α/-α, --/αα Hb H disease Three gene deletion α-/-- Hydrop fetailis Four gene deletion --/-- 13 α THALASSEMIA GENETIC POLYMORPHISM Genetic polymorphism of alpha thalassemia. The orang boxes represent normal genes, and blue boxes shows gene deletions or less frequently dysfunctional genes 14 ALPHA THALASSEMIA Silent carrier [-α/αα] These individuals have no anemia, splenomegaly or any detectable clinical symptoms RBCs are not microcytic, and Hb A2 and Hb F are normal. During the newborn period, small amounts (2%) of Hb Bart (γ4) can be seen by electrophoresis After disappearance of hemoglobin Bart’s, no recognizable hematologic abnormality is seen except for the borderline low MCV of red cells Salient carrier of alpha thalassemia is diagnosed at molecular level with amplification refractory mutation system (ARMS-PCR) Polymerase Chain Reaction technique 15 ALPHA THALASSEMIA α thalassemia trait [-α/-α, --/αα] α thalassemia trait is usually asymptomatic disease and patients generally lead a normal life They may, however persist mild anemia with microcytosis, hypochromia, slightly jaundiced and their spleen may be a little larger than normal Women with α-thalassemia minor may become more anemic during pregnancy The mean corpuscular volume (MCV) and mean corpuscular haemoglobin (MCH) are low and the red cell count is over 5.5 × 10^12/L. Haemoglobin electrophoresis is normal and DNA analysis is needed 16 to be certain of the diagnosis ALPHA THALASSEMIA Hb H disease [α-/--] Children with hemoglobin H disease present with variable degree of microcytic and hypochromic anemia Anemic patients may develop the physical and bony characteristics of thalassemia major Hepatomegaly and splenomegaly are common in these patients These patients rarely need blood transfusion Adults with hemoglobin H disease may have hemoglobin H from 540%, with the remainder being mostly hemoglobin A, with small amount of hemoglobin A2 and hemoglobin Bart’s Infants who later develop hemoglobin H disease usually have 1927% hemoglobin Bart’s at birth with the remainder composed of hemoglobin F and A 17 ALPHA THALASSEMIA Pathophysiology of Hb H disease Pathophysiology of Hb H disease in adult. During fatal life, similar syndrome but with accumulation of Hb Barts. 18 ALPHA THALASSEMIA Hydrops fetalis [--/--] In this type of α thalassemia, no α chains are produced Because of the complete suppression of α globin gene no Hb F or Hb A is produced At birth, these infants are severely anemic and edematous and present with ascites, marked hepatomegaly and splenomegaly Affected fetuses are usually born prematurely and death of the baby takes place either in utero due to fetal hypoxia or very soon after birth Hb electrophoresis reveals predominantly Hb Bart, with a smaller amount of Hb H A minor component identified as Hb Portland migrating in the position of Hb A is also seen. Normal Hb A and Hb F are totally absent 19 ALPHA THALASSEMIA Pathophysiology of Hydrops fetalis 20 BETA THALASSEMIA Beta thalassemia syndrome is an autosomal recessive genetic disorder characterized by absence or reduced β-chains synthesis due to mutations in beta gene Currently more then 400 beta gene mutations have been identified Unlike α‐thalassemia, the majority of genetic lesions are point mutations rather than gene deletions. These mutations may be within the gene complex itself or in promoter or enhancer regions It has been determined that five mutations [codon 41/42 (-TTCT), codon 17 (A>T), nt-28 (A>G), IVS II-654 (C>T) and IVS I-5 (G>C)] usually account for more than 90% of the cases of β-thalassemia in the world β-thalassemia syndrome further divided into β-thalassemia trait or 21 minor, β-thalassemia intermedia and β thalassemia Major β THALASSEMIA GENETIC POLYMORPHISM Genotype Genetic description Phenotype βο/βο Homozygous Major β+/β+ Homozygous Major or Intermedia βο/β+ Heterozygous Major or Intermedia βο/β Heterozygous Intermedia or minor β+/β Heterozygous Minor 22 BETA THALASSEMIA Beta Thalassemia trait or minor Beta thalassemia trait (BTT) is a heterozygous, autosomal recessive inherited disorder characterized by mild deficiency of beta globin chain synthesis leads to compensatory hemolytic disease It is usually asymptomatic as sufficient amount of hemoglobin A is produced which prevents the adverse effects of hemolytic process This may lead to moderate anemia when coexists with iron, B12/folate deficiencies and severe infections or noted in pregnancy Itself it is not lethal, patients generally lead a normal life but when both parents are beta thalassemia trait there is 25% probability to give birth a child with beta thalassemia major (homozygous) at each pregnancy 23 BETA THALASSEMIA Beta Thalassemia trait (BTT) laboratory features The Hb level averages 1 or 2 g/dL lower than that seen in normal persons of the same age and gender Total RBC count show relative erythrocytosis usually >5.0 millions/µl Red cell indices shows decrease MCV and MCH with normal MCHC and RDW Peripheral blood film reveals microcytic hypochromic red blood cells, occasional target cells, polychromasia and basophilic stippling Reticulocytes count is slightly elevated Raised hemoglobin A2 level (>3.5%) on hemoglobin electrophoresis is diagnostic findings 24 BETA THALASSEMIA Beta Thalassemia trait (BTT) laboratory features 25 BTT blood film with Giemsa stain Raised HbA2 consistent with BTT BETA THALASSEMIA Beta Thalassemia intermedia β thalassemia intermedia is an entity of β thalassemia in which the clinical symptoms are intermediate between β thalassemia major and β thalassemia minor It is characterized by mild to moderate anemia (7-10 g/dl) which is mostly transfusion not required and occasionally transfusion dependent It can be inherited as homozygous (β+/β+) or compound heterozygous (βο/β+ or βο/β) state Approximately 10% of patients with homozygous β-thalassemia exhibit a phenotype characterized by intermediate hematologic severity 26 BETA THALASSEMIA Beta Thalassemia intermedia Splenic and liver enlargement is also a salient feature of beta thalassemia intermedia β thalassemia intermedia is belong from non transfusion dependent beta thalassemia group as shown in table 7.3 27 BETA THALASSEMIA Beta Thalassemia intermedia laboratory features Complete blood counts shows moderate anemia with decreased MCV, MCH and mean MCHC RDW is either normal or increased depending upon the clinical severity of anemia Morphology shows microcytic and hypochromic red cells, target cells, fragmented cells and tear drop cells Reticulocytosis (>5%) with normal WBCs and platelets counts Hemoglobin electrophoresis shows decreased amount of Hb A, variable 28 amount of Hb A2 (5-10%) and usually increased level of Hb F (30-75%). BETA THALASSEMIA Beta Thalassemia Major Beta thalassemia major is an autosomal recessive inherited disorder characterized by complete absence of beta globin chain synthesis leads to severe transfusion dependent anemia, hepatosplenomegaly and bone deformities It can be inherited as homozygous (β+/β+ or βο/βο) or compound heterozygous (βο/β+) state Beta thalassemia major is also known as Cooley’s anemia 29 BETA THALASSEMIA Beta Thalassemia Major pathophysiology In β thalassemia major there is complete absence of β globin chain, however α globin chain production is not affected As a result, free α globin chains accumulate in the developing red cells to form α globin chain tetramers which precipitate out as intracellular inclusions These inclusions are pitted out by macrophages of reticuloendothelial system that results in premature hemolysis and hypersplenism Hemolysis lead to transfusion dependent anemia, increased indirect bilirubin level, gall stones and enhance iron absorption 30 BETA THALASSEMIA Beta Thalassemia Major pathophysiology Anemia causes increase erythropoietin secretion from kidney that leads to erythroid hyperplasia in the bone marrow This results in expansion of medullary cavities of bones specially skull bones Hepatosplenomegaly and bone deformities is induced due to extra medullary erythropoiesis Continues transfusion lead to iron overload, transfusion reaction and transfusion transmitted diseases Iron overload toxicity causes vital organ failure i-e cardiac arrest, endocrine dysfunction, cirrhosis etc. 31 BETA THALASSEMIA Beta Thalassemia Major pathophysiology 32 BETA THALASSEMIA Clinical features of beta thalassemia major At birth, patients with β thalassemia major are asymptomatic due to increased concentration of hemoglobin F Severe anemia becomes apparent at 3–6 months after birth when the switch from γ‐ to β‐chain production should take place Typically the infant presents in the first year with irritability, growth retardation, pallor and a swollen abdomen caused by enlargement of the liver and spleen Enlargement of the liver and spleen occurs as a result of excessive red cell destruction, extra medullary hematopoiesis and later because of iron overload 33 BETA THALASSEMIA Clinical features of beta thalassemia major Expansion of bones caused by marrow hyperplasia leads to a thalassemic facies To thinning of the cortex of many bones with a tendency to fractures and skull with a ‘hair‐on‐end’ appearance on X-ray Repeated blood transfusions lead to accumulation of iron in various organs Myocardial hemosiderosis leads to arrhythmias and cardiac failure Hemosiderosis of liver and spleen leads to abnormal function of liver and spleen Iron deposition in endocrine organs leads to hypothyroidism, hypoparathyroidism and growth hormone deficiency. Gonadal dysfunction is associated with delayed puberty and infertility 34 BETA THALASSEMIA Clinical features of beta thalassemia major Infections occur frequently in infancy, without adequate transfusion, anemia predisposes to bacterial infections Pneumococcal, Haemophilus and meningococcal infections are likely if splenectomy has been carried out Yersinia enterocolitica occurs, particularly in iron‐loaded patients being treated with deferoxamine Iron overload itself also predisposes to bacterial infection, e.g. Klebsiella, and to fungal infection 35 BETA THALASSEMIA Clinical features of beta thalassemia major Liver disease in thalassemia is most frequently a result of hepatitis C, hepatitis B and iron overload may also cause liver damage Hepatocellular carcinoma incidence is increased in those with iron overload and chronic hepatitis B or C Human immunodeficiency virus (HIV) has been transmitted to some patients by blood transfusion Osteoporosis may occur in well‐transfused patients. It is more common in diabetic patients with endocrine abnormalities 36 BETA THALASSEMIA 37 BETA THALASSEMIA Hematological laboratory findings of beta thalassemia major Complete blood count and peripheral blood smear examination Hb: decrease MCV: decrease TLC: normal/increase MCH: decrease Platelets: normal MCHC: decrease RBC count: decrease RDW: markedly increase HCT: decrease Retic count: increase Peripheral blood film reveals marked anisocytosis, pokilocytosis, polychromasia and severe hypochromia Nucleated RBCs are present Target cells and fragmented red cells are characteristic findings 38 BETA THALASSEMIA Hematological laboratory findings of beta thalassemia major Coarse basophilic stippling can be easily observed Tear drop cells and occasionally elliptocytes may be seen Howell jolly bodies are visible Increased reticulocyte count is a consistent feature of β thalassemia major White cell count may be normal or increased in number showing toxic changes due to infection Usually, platelet count remains normal 39 BETA THALASSEMIA 40 BETA THALASSEMIA Hematological laboratory findings of beta thalassemia major Bone marrow examination Bone marrow examination is not indicated in β thalassemia major However, bone marrow shows erythroid hyperplasia with marked ineffective erythropoiesis Myeloid and megakaryocytic cell lines show normal maturation Increased iron stores can be demonstrated by Perl’s/Prussian blue or iron stain 41 BETA THALASSEMIA Diagnostic laboratory investigations of Beta Thalassemia major Hemoglobin Electrophoresis Hemoglobin electrophoresis shows marked elevation of hemoglobin F (10-98%), hemoglobin A2 (≥3.5%) and low hemoglobin A level Hemoglobin F can also be demonstrated by acid elution test or by alkali denaturation test Heinz bodies can be demonstrated by supra vital stains Molecular analysis DNA analysis specifies the genetic mutation which determines the severity of the disease. 42 BETA THALASSEMIA Hemoglobin Electrophoresis Methods 1. Manual method Agrose gel Electro Cellulose acetate Electro 2. Automated method Capillary Electro HPLC 43 BETA THALASSEMIA Biochemical laboratory findings of beta thalassemia major Serum Iron Profile Serum total iron Markedly increased Serum ferritin Increased TIBC(total iron binding capacity) Saturated Transferrin saturation % Increased Other biochemical investigations Unconjugated bilirubin and LDH Raised Haptoglobin and serum folate Decreased 44 Urobilinogen Increased Thank You Any Question.? 45 SICKLE CELL ANEMIA Awal Mir M.Phil MLS SICKLE CELL ANEMIA Sickle cell disorder is autosomal co-dominant inherited hemoglobinopathies characterized by the production of abnormal sickle hemoglobin (HbS) lead to blockage of microvasculature, organ infarction and moderate to severe hemolytic anemia. Hemoglobin S is produced when nonpolar valine is substituted for polar glutamic acid at 6th position in the β chian In homozygous state the individual inherits a double dose of the abnormal gene (β s /β s ) that codes for hemoglobin S lead to sickle cell anemia (severe form) Individuals heterozygous for HbS (β s /β) produce more than 50% of HbA while HbS is mostly 25-35% 3 SICKLE CELL ANEMIA Beta Chain (142 amino acids) 1, 2, 3, 4, 5 6 7, ……142 4 SICKLE CELL ANEMIA 5 PATHOPHYSIOLOGY Hemoglobin S is soluble and usually causes no problem when properly oxygenated. When the red cells containing HbS passes through microcirculation of spleen, alteration in the solubility of hemoglobin occurs. Hb S (Hb α2 β2 S ) is insoluble and forms crystals when exposed to low oxygen tension. Deoxygenated sickle hemoglobin polymerizes into long fibres, each consisting of seven intertwined double strands with cross‐linking. This results in sickling of red cells which can be reversed in good O2 tension 6 PATHOPHYSIOLOGY Repeated sickling and unsickling of red cells causes membrane damage and ultimately they become permanently sickled Permanently sickled cells there is accumulation of calcium, lose potassium & water and become rigid These sickle cells get trapped in the splenic macrophage that results in chronic extravascular hemolysis As these cells are less deformable in circulation due to mechanical fragility they get destroyed rarely leading to intravascular hemolysis 7 PATHOPHYSIOLOGY This leads to hypoxia, painful crises and infarction of the organs Sickle cells also aggregate in vessels that result in increased blood viscosity, vascular stasis and ischemia Factors that influence the sickling phenomenon are intracellular concentration of HbS, increased MCHC, reduced oxygen tension, acidosis, cold temperature and association with thalassemia and other hemoglobinopathies. 8 CLINICAL FEATURES Anemia: It is due to extravascular hemolysis and secondary to folate &B12 deficiency as a result of ineffective erythropoiesis. Gall stones is due to chronic hemolysis and hyperbilirubinemia Iron overload and persistent oxygen deficit lead to cardiac hypertrophy, cardiac enlargement, and eventually congestive heart failure Vaso-occlusive crisis: Sickled cells is less deformable, difficulty squeeze, fragile, trapped and tend to aggregate in the microvasculature lead to vaso-occlusive crisis and necrosis 9 CLINICAL FEATURES Vaso-occlusive crisis: It is present with tissue pain, ischemia, necrosis may lead to tissue damage. Acute splenic sequestration: In young children sudden splenic pooling of sickled erythrocytes. Acute, painful enlargements of the spleen, caused by intra-splenic trapping of red cells. Thrombocytopenia can also occur. Hypovolemia and shock is follow. At one time, splenic sequestration was the leading cause of death in infants 10 CLINICAL FEATURES Prone to bacterial infections: Bacterial pneumonia and meningitis is the most common infections. The reasons for this increased susceptibility to infection are not fully understood but could be related to functional asplenia, impaired opsonization and abnormal activation of complement system. Acute chest syndrome: Resembling to pneumonia, most common cause of death in children. Clinical findings include cough, fever, chest pain, dyspnea, chills, wheezing, and pulmonary infiltrates. Hemoglobin & oxygen saturation decrease. The etiology of acute achest syndrome is not clear 11 CLINICAL FEATURES Iron overload: Iron overload or hemosiderosis is due to increased hemolysis, increase iron absorption and transfusion therapy. Hepatic fibrosis and cirrhosis related to hemosiderosis can occur. Other complications: Auto-splectomy, renal failure, proteinuria, hematuria and glomerular sclerosis are common in sickle cell anemia. In pregnancy, there is increase risk of abortion, intrauterine growth restriction (IUGR) and prematurity still birth. 12 LAB DIAGNOSIS Complete blood count & peripheral smear examination Sickling Test Hemoglobin Electrophoresis Molecular studies 13 LAB DIAGNOSIS Peripheral smear examination shows normocytic and normochromic red cells, sickle cells, numerous target cells, fragmented cells, polychromasia and nucleated red cells. Howell-Jolly bodies and basophilic stippling can be demonstrated in case of autsplenectomy in patients. Reticulocyte count is usually raised 14 LAB DIAGNOSIS Peripheral smear examination 15 LAB DIAGNOSIS Sickling test: A sample of venous blood or capillary blood may be collected for this test. Mixing blood with the reducing agent, sodium metabisulphite, will induce sickling in susceptible cells. Sickled RBC Normal RBC 16 LAB DIAGNOSIS Hemoglobin Electrophoresis: In homozygous state, HbS constitutes about 80-90% and HbF 10-30% with minimal or no HbA. HbA2 in these patients may be slightly increased with a mean of 3.4% In sickle cell trait, HbA constitutes 40% to 60%, HbS 25 to 35% and usually elevated HbA2 level 17 Thank You Any Question.? 18 GLUCOSE 6 PHOSPHATE DEHYDROGENASE DEFICIENCY ANEMIA Awal Mir M.Phil MLS G6PD DEFICIENCY ANEMIA Glucose 6 Phosphate Dehydrogenase (G6PD) deficiency is a multi ethnic sex- linked recessive, inherited disorder lead to chronic hemolytic anemia. It is the most frequent red cells enzymopathy of the world. G6PD enzyme protects RBC from destruction in response to oxidative damage. G6PD catalyzes the first and rate limiting step in the pentose phosphate pathway to generate nicotinamide adenine dinucleotide Phosphate (NADPH) that is subsequently utilized in oxidative stress in RBC 3 G6PD DEFICIENCY ANEMIA 4 HISTORICAL BACKGROUND G6PD deficiency was first time discovered in 1956 by Alving and his colleagues In the search of hemolytic anemia occurring in some individuals treated with Primaquin (Anti-malarial) in Blacks. Later it was known that G6PD deficiency not only occurred in Africans (Blacks) but was prevalent worldwide. Recently it has been documented that approximately 400 million peoples are G6PD deficient globally 5 G6PD DEFICIENCY G6PD enzyme activity is controlled by G6PD gene located on the long arm of the Sex linked chromosome [Xq28] The G6PD gene is X-lined recessive in its pattern of inheritance and consists of 13 exons and 12 introns that encode a 515 amino acids monomer Active G6PD enzyme exists in homo-dimmers or tetramers Males are hemizygous and have only one gene copy for G6PD on X chromosome. 6 G6PD DEFICIENCY The G6PD gene in males can be expressed normally or abnormally to make them G6PD diffident. Females contain two copies of G6PD genes on each Sexchromosomes and so they can be normal, heterozygous or rarely homozygous Heterozygous females are the carrier of defective gene has normal, intermediate or very low G6PD enzyme activity 7 G6PD DEFICIENCY G6PD deficiency is due to one or more mutations in the G6PD gene Leading to functional variants of the proteins resulting in different phenotypes. More than 400 mutations have been reported so far. Many G6PD enzyme deficient individuals are unaware their G6PD status and asymptomatic in their whole life 8 G6PD DEFICIENCY G6PD defiant individuals lead to onset of acute hemolysis, when red blood cells exposed to oxidative stress due to some factors Such as drugs (Primaquin), foods (Favabean, Pumpkin) and infections (Hepatitis A & B, Cytomegaly virus, Pneumonia and typhoid) . Some clinical disorders have been reported which can cause hemolysis in G6PD 9 G6PD DEFICIENCY Such as diabetes, Myocardial infarction and strenuous physical exercise. Several medicines interlinked to acute hemolysis in G6PD deficient individuals But it is difficult to specify medicines responsible for hemolytic crisis in G6PD deficient individuals as shown in the table 10 G6PD DEFICIENCY 11 G6PD VARIANTS 12 CLINICAL FEATURES Clinically G6PD deficient individual complain of fatigue, back pain, anemia, jaundice and black urine Acute, acquired hemolytic anemia (episodic), including favism Hereditary (congenital) nonspherocytic hemolytic anemia (chronic) Neonatal hyperbilirubinemia with jaundice 13 LAB DIAGNOSIS CBC and peripheral blood film examination Peripheral blood film morphology shows normocytic normochromic red blood cells with fragmented RBCs (typically bite and blister cells), spherocytes, polychromasia and nucleated RBCs may be present Elevated reticulocytes count 14 LAB DIAGNOSIS 15 LAB DIAGNOSIS G6PD Assays Several screening tests for G6PD are available such as dyecolorization test, monospotfluerocent spot test, methhemoglobine reduction test and farmazon test. G6PD Gene mutation variants and females carrier status can be identified by Polymerase chain reaction- restriction fragment length polymorphism (PCR-RFLP) or Denaturing high performance liquid chromatography (DHPLC) 16 Thank You Any Question.? 17 HEREDITARY SPHEROCYTOSIS Awal Mir M.Phil MLS HS INTRODUCTION Hereditary spherocytosis is a genetic disorder of red cell membrane characterized by defected cytoskeletal proteins lead to chronic hemolytic anemia and spherocytosis in peripheral blood. Spherocyte is abnormal shape of red blood cells, smaller in size spherical or round in shape with no central pallor area and decrease surface to volume ratio. 3 RBC MEMBRAIN FUNCTIONS Maintain the normal discoid shape of red blood cell Preserved cell deformability and fragility characteristics Retain selective permeability Maintain red blood cell survival (90-120 days) 4 NORMAL RBC MEMBRAIN STRUCTURE Composition of red blood cell membrane: 1. Proteins: 50% 2. Lipids: 40% 3. Carbohydrates: 10% 5 DEFECTS IN RBC MEMBRANE Membranopathes is genetic defect of cytoskeletal protein genes that involved vertical and horizontal interactions 6 INTERACTIONS OF MEMBRANE PROTEIN AND LIPIDS Vertical Interactions It is perpendicular to red cell membrane This interactions is between skeletal framework to integral proteins and lipids of membrane These interactions stabilize the lipid bilayer of the membrane Such as Ankirin, Band 3 ,Glycoporin 3, and Protein 4.2 etc 7 INTERACTIONS OF MEMBRANE PROTEIN AND LIPIDS Horizontal Interactions Parallel to the plane of the membrane Important in the formation of the stress-supporting skeletal protein frame that provides mechanical stability This causes cell fragmentation with formation of poikilocytes Proteins include spectrin and actin 8 SPHEROCYTES FORMATION MECHANISMS 9 SPHEROCYTES FORMATION IN HS 10 HS PATHOPHYSIOLOGY 11 HS CLINICAL FEATURES 1. Mild type HS 20 to 30 percent have of cases. Compensatory hemolytic process with no anemia Little splenomegaly or jaundice, Normal hemoglobin levels Mostly asymptomatic but symptomatic when coexist with viral infections or during pregnancy 12 HS CLINICAL FEATURES 2. Moderate type HS 60 to 75 percent of HS cases with moderate type. Moderate anemia Have high reticulocyte counts, Elevated serum bilirubin concentrations. Mild to moderate splenomegaly 13 HS CLINICAL FEATURES 2. Severe type HS Less frequent (5%) Severe Anemia with marked hemolysis Marked jaundiced (17-70 micro mole/L) Marked splenomegaly Gall bladder stones Aplastic crisis, hemolytic crisis and megaloblastosis due to folic acid deficiency are some of the other characteristic of this disease. 14 LABORATORY DIAGNOSIS CBC and Peripheral smear examination Biochemical tests Osmotic Fragility test Membrane protein SDS-PAGE Molecular studies 15 LABORATORY DIAGNOSIS Complete blood count and peripheral blood smear examination Hb: Decrease RBC: Decrease HCT: Decrease MCV: Normal MCH: Normal MCHC: Increase Peripheral blood smear shows polychromasia and variable number of spherocytes with normocytic and normochromic red cells. WBCs and platelets are normal in number and morphology. Nucleated RBC, Howell-Jolly bodies may appear in large numbers in hemolytic crisis 16 LABORATORY DIAGNOSIS 17 LABORATORY DIAGNOSIS Biochemical Tests Serum Indirect Bilirubin: Increased Urine Urobilinogen: Increased Serum LDH: Increased Haptoglobin and Hemopexin levels: Decreased 18 LABORATORY DIAGNOSIS Osmotic Fragility test When an erythrocyte is placed in a hypotonic sodium chloride-(NaCl) solution, a net influx of solvent (water) into the cell-will occur If the cell size reaches a certain point, the cell membrane will become leaky and hemoglobin will diffuse out (hemolysis) If the NaCl solutions hypotonic enough, the cell will rupture. The degree of hemolysis can be measured by determining the absorbance of the supernatant using a spectrophotometer 19 LABORATORY DIAGNOSIS Normal Abnormal 20 Thank You Any Question.? 21 IRON METABOLISM AND IRON DEFICIENCY ANEMIA Awal Mir M.Phil MLS IRON Iron is one of the most common elements in the Earth’s crust. Iron is an essential element required for energy production, oxygen use, and cellular proliferation. Iron must be bound to protein compounds to fulfill these functions. Each cell in the body needs iron, not too much and not too little. Iron is ionized compound, overload lead to iron toxicity resulting organ damage. Conversely, if too little iron is available, the synthesis of physiologically active iron compounds is limited, and critical 3 metabolic processes are inhibited. IRON CONTAINING COMPOUNDS 4 SOURCES OF IRON Sources of Iron Non-Dietary iron Source (85%) Dietary iron (10-15 %) Plant/non-heme source (Ferric form) • • • • • Apple Pear Spinach Date Bean Animal/heme source (Ferrous form) • • • • Red meat Beef or chicken Liver Fish egg Erythropoiesis Recycling Globin Heme Hemoglobin Normal RBC destruction 5 DAILY IRON REQUIREMENTS 6 IRON CYCLE 7 IRON DISTRIBUTION Most body iron is present in haemoglobin in circulating red blood cells (1.7-2.4 g). The macrophages of the reticuloendotelial system store iron released from haemoglobin as ferritin and hemosiderin (0.3-1.5 g) Myoglobin is also contain 0.15 g iron. Transporting iron (transferrin): 0.003-0.004 g. Heme enzymes: 0.02-0.02 g. Approximately 1 to 2 mg iron losses each day in urine, faeces, skin and nails and in menstruating females as blood and 1 to 2 8 mg iron is absorb from diets in duodenum. DIETARY IRON ABSORPTION Dietary ingredients ingest about 15 mg iron of which only 10% will be absorbed, giving him 1.5 mg/day of iron that can be used for red cell production or stored in the reticuloendothelial system (RES). HCL in stomach detach iron from food particles. Iron is absorbed in upper part of the duodenum in slight acid medium. Iron is absorbed in ferrous form. Ferric form is first converted into ferrous form by action of ferrireductase enzyme present on brush border of enterocyte. Transportation of iron from GI tract to bone marrow via transferrin 9 DIETARY IRON ABSORPTION 10 DIETARY IRON ABSORPTION 11 IRON TRANSPORTATION AND STORAGE Transferrin is iron containing transporter protein composed of appotransferrin and two iron molecules. Iron is absorbed in ferrous form and transported in ferric form so it is first converted into ferric form by the action of oxygenase enzyme before to bind with appotransfferin. 1 gram of transferrin binds 1.4 mg of iron (total iron binding capacity). Iron is utilize in bone marrow for the developing normoblast for use of hemoglobin synthesis or its is store in macrophages of reticuloendothelial system in the form of ferritin or hemosidrin. 12 IRON UTILIZATION IN B.M 13 IRON STORAGE Iron is stored mainly in the liver in reticuloendothelial system as Hemosiderin Ferritin Hemosiderin is the major long term storage form of iron ; release slowly, Ferritin is the primary storage form of soluble iron ;release readily at time of need. 14 FERRITIN Iron storage protein In humans, it acts as a buffer against iron deficiency and iron overload Consists of: Apoferritin – protein component Core- ferric, hydroxyl ions and oxygen Largest amount of ferritin-bound iron is found in: Liver hepatocytes (majority of the stores) BM Spleen Excess dietary iron induces increased ferritin production Partially digested ferritin= HAEMOSIDERIN- insoluble and can be detected in tissues (hepatocytes) using Perl’s Prussian blue stain 15 HEMOSIDRIN Water insoluble protein iron complex Visible by light microscope It has higher iron to protein ration up to 37% than ferritin up to 20% Formed by partial digestion of ferritin aggregates by lysosomal enzymes. Hemosiderin is present predominately in macrophages rather than hepatocytes. 16 TRANSFERRIN Transports iron from palsma to erythroblast Mainly synthesized in the liver Fe3+ (ferric) couples to Tf Apotransferrin = Tf without iron Contains sites for max 2 iron molecules Synthesis is inversely proportional to iron store 17 PROTEINS INVOLVED IN IRON HOMEOSTASIS 18 IRON METABOLISM DISORDERS IDA = Iron deficiency anemia, ACD = Anemia of chronic disease, SA = Sidroblastic anemia. 19 IRON DEFICIENCY ANEMIA It is a clinical condition characterized by low level of hemoglobin due to depletion of iron stores in the body lead to mild to severe anemia. 20 IRON DEFICIENCY ANEMIA IDA is the most common nutritional deficiency anemia in the world. Globally about 20% of women, 90% of pregnant women and 3% of men present with iron deficiency anemia. On the bases of severity IDA is divided into 3 categories. Mild anemia (With hemoglobin level 9-12 g/dl) Moderate anemia (With hemoglobin level 6-9g/dl) Severe anemia (With hemoglobin level <6g/dl) 21 ETIOLOGY OF IDA Increased Iron demand/Utilization Pregnancy Infancy Adolescence Blood Loss/iron loss (1 ml blood loss = 0.5 mg iron loss) Gastrointestinal Tract (hemorrhoid, hook worm, gastritis) Menstrual Blood Loss Nose bleeding (Epistaxis) Urinary Blood Loss (hematuria and hemoglobinemia and hemosidrinuria) Mal-absorption Tropical Sprue Gastrectomy Chronic atrophic gastritis Poor iron diet intake 22 PATHOPHYSIOLOGY OF IDA Iron deficiency anaemia develops in three stages Iron depletion: Iron stores decreases (low ferritin) but no anemias and erythrocyte morphology is normal. Elevated RDW. Iron deficient erythropoiesis: There is insufficient iron to insert into the protoporphyrin ring to form heme. Serum iron is also depleted but no anaemia and hypochromia. Erythrocytes may became slightly microcytic Iron deficiency anaemia: A long-standing negative iron flow eventually leads to the last stage of iron deficiency. All laboratory tests for iron status become markedly abnormal. Classic microcytosis and hypochromia 23 CLINICAL FEATURES Generalize sign and symptoms • • • • • Pallor Fatigability Dizziness Headache Shortness of breath Specific sign and symptoms • • • • Koilonychias (Spoon shaped nails) Glossitis (Inflammation of the tongue) Angular cheilosis (Ulcerations corner of the mouth) Pica syndrome (Appetite for non food substances such as clay) Geophagia (clay eating tendency), Phagophagia (ice eating tendency), Amylophagia (Starch eating tendency) 24 CLINICAL FEATURES 25 LABORATORY DIAGNOSIS Complete blood count and peripheral blood smear examination Hb: decrease MCV: decrease TLC: normal MCH: decrease Platelets: normal/increase MCHC: decrease RBC count: decrease RDW: increase HCT: decrease Retic count: decrease Peripheral blood film reveals anisopoikilocytosis with microcytosis, hypochromia, eliptocytosis (pencil cells) and few tear drop and target cells. WBCs are normal. Platelets are high (reactive thrombocytosis) on film examined. 26 LABORATORY DIAGNOSIS 27 LABORATORY DIAGNOSIS Serum Iron Profile Serum total iron Decreased Serum ferritin Decreased TIBC(total iron binding capacity) Increased Tansferrin saturation % Decreased Bone marrow examination (Not required for IDA diagnosis) Hypercellur marrow with micronormoblastic erythropoiesis. Myelopoiesis is active with all stages of maturation. Megakaryopoiesis is normal. Iron stain shows no stainable iron seen. 28 LABORATORY DIAGNOSIS 29 Thank You Any Question.? 30 APLASTIC ANEMIA Awal Mir M.Phil MLS APLASTIC ANEMIA Aplastic anemia is a disease of pluripotential hematopoietic stem cells characterized by pancytopenia in the peripheral blood and fatty replacement of hematopoietic tissues in the bone marrow The mature blood cells that are produced in aplastic anemia usually appear normal Pancytopenia is a condition in which trilineage of hematopoiesis is reduced lead to anemia, leukopenia and thrombocytopenia 3 PANCYTOPENIA ANEMIA CRITERIA Hemoglobin Level = < 10.0 g/dL Total Leukocyte count: 4.0 x 10^9/L OR Normal TLC with neutropenia Platelet count: 100 x 10^9/L 4 APLASTIC ANEMIA CLASSIFICATION Classification and etiology of Aplastic Anemia (AA) Inherited AA Acquired AA Secondary Chemical Agents Benzene Insecticides Hair dye Carbontetrachloride Chemotherapeutics Arsenic Primary-Idiopathic Drugs Chloramphenicol Phenylbutazone Gold compounds Sulfa drugs Antihistamines Antithyroid Tetracyclines Penicillin Fanconi Anemia Chronic Hemolytic Infections Ionizing radiation anemias Parovirus H. Spherocytosis s Infectious mononucleosis Sickle cell anemia Infectious hepatitis PNH Measles Influenza Auto immune disease SLE 5 ACQUIRED APLASTIC ANEMIA Idiopathic aplastic anemia The majority of cases of aplastic anemia cannot be linked to an environmental factor and are referred to as idiopathic. It is possible that previous exposure to an unrecognized agent or event could be responsible for stimulating the immune system 6 ACQUIRED APLASTIC ANEMIA Chemicals induced aplastic anemia Large number of substance both in the household and in the industry have been incriminated in the development of aplastic anemia. Most of these substances have a benzene ring in their structure 7 ACQUIRED APLASTIC ANEMIA Radiation induced aplastic anemia High dose radiations to the bone marrow cause variable degree of marrow aplasia The damage is proportionate to the dose of radiations Large doses administered to any individual will completely ablate the bone marrow 8 ACQUIRED APLASTIC ANEMIA Hepatitis-associated marrow aplasia Recently an association has been established between marrow aplasia and non-A, non-B type infectious hepatitis. It commonly affects young male; 75 % of the affected patients are under 20 years of age. Marrow hypoplasia mostly appears within 2 months after the onset of jaundice. The outcome is usually fatal 9 INHERITED APLASTIC ANEMIA Congenital Aplastic Anemias (Fanconi anemia) Fanconi type anemia is inherited as autosomal recessive trait They account for almost 20-30% of all cases of aplastic anemia in children It is commonly associated with skeletal abnormalities, growth retardation, microcephaly and pigmentary dermatoses Nearly 10% of the patients develop acute non-lymphoid type of leukemia 10 INHERITED APLASTIC ANEMIA Congenital Aplastic Anemias (Fanconi anemia) This type of anemia frequently displays non-specific chromosomal breaks which are best demonstrated in tissue cultures of peripheral blood lymphocytes Clinical manifestations are those of slowly progressive anemia with features of systemic abnormalities If not treated, follow a progressively downhill course and most patients die within 2 years after the diagnosis is made 11 CLINICAL FEATURES 12 CLINICAL FEATURES 13 CLINICAL FEATURES 14 CLINICAL FEATURES 15 CLINICAL FEATURES 16 LABORATORY DIAGNOSIS Complete blood count and peripheral blood smear examination Bone Marrow examination Cytogenetic 17 LABORATORY DIAGNOSIS Complete blood count and peripheral blood smear examination Hb: Decrease MCV: Normal TLC: Decreased MCH: Normal Platelets: Decreased MCHC: Normal RBC count: Decreased RDW: Normal HCT: Decreased Retic count: Decreased Peripheral blood film reveals normocytic and normochrmic red blood cells with occasional nucleated RBCs. Marked leukopenia and neutropenia with normal lymphocytes. Platelets are reduced on blood film examined. 18 LABORATORY DIAGNOSIS Bone Marrow examination Bone Marrow aspiration reveals Hypocellular marrow with reduced erythropoiesis, Myelopoiesis and Megakaryopoiesis. Lymphocytes and plasma cells are normal. Iron storges are increased. Bone marrow trephine biopsy reveals hypocellular marrow with replacement of adipocytes. Lymphocytes and plasma cells are normal. There is no evidence of leukemia/lymphoma or granuloma identified on bone marrow slid examination 19 LABORATORY DIAGNOSIS Fanconi Anemia diagnosis Genetics: Increased sensitivity of the cells to chromosomal damage by DNA cross linking agents. 13 genes are responsible IV54 mutation, is associated with multiple dysmorphism, severe pancytopenia, higher incidence of AML 20 LABORATORY DIAGNOSIS Bone Marrow examination Plastic Marrow Normal Marrow 21 Thank You Any Question.? 22 ABO BLOOD GROUP SYSTEM Awal Mir M.Phil MLS BLOOD GROUP SYSTEMS A total of 30 blood group systems have been described All blood systems are clinical significant and each system is a series of red cell antigens, determined either by a single genetic locus or by very closely linked loci. ABO and Rh blood group systems are known as major blood group systems while the rest is minor blood group systems A numerical catalogue of red cell antigens is also being maintained by an International Society of blood transfusion (ISBT) 3 BLOOD GROUP SYSTEMS Blood group systems Major Blood group systems • ABO Blood group system • Rh Blood group system Minor Blood system • Kidd • Kell • MNs • Duffy • Lewis • P • MNS • I • etc. 4 ISBT CLASSIFICATION 5 ISBT CLASSIFICATION 6 ABO BLOOD GROUPS SYSTEM Historical Background An Austrian Scientist Karl Landsteiner discovered the ABO / ABH blood group system in 1901 Alexis Carrel developed a serological technique for transfusion in 1908 In 1926 it was found that A and B antigens were not only present on red cells but they were also present in soluble form in saliva 7 ABO BLOOD GROUPS SYSTEM Historical Background In 1930, Putkonen noted that a person could be either secretor or non-secretor It is now known that ABH blood group antigens (A, B and H) are found on red blood cells, lymphocytes, platelets, tissue cells, body fluids and secretions ABO blood group system is not only important for blood transfusion but also important in tissue and bone marrow transplant 8 ABO BLOOD GROUPS SYSTEM Historical Background Karl Landsteiner and his five co-workers began mixing each others red cells and serum together and unintentionally performed the first forward and reverse ABO groupings Currently ABO forward and reverse grouping tests must be performed on all donors and patients 9 ABO BLOOD GROUPS SYSTEM Landsteiner Rules: If an antigen is present in the RBC’s of an individual, the corresponding antibody must be absent from the plasma If an antigen is absent in the RBC’s of an individual, the corresponding antibody must be present from the plasma For example, if the individual has A antigens only on their red blood cells, there will be an “expected” naturally occurring anti-B antibody in their serum 10 ABO BLOOD GROUPS SYSTEM Major ABO blood groups: 11 INHERITANCE OF ABO BLOOD SYSTEM ABO blood group gene is located on chromosome no 9 and composed of 7 exon. The inheritance was first time described by Bernstein in 1924 He demonstrated that one gene of ABO inherited from each parent as a co-dominant pattern and have three allele genes A, B and O The O gene is considered an amorph, as no detectable antigen is produced 12 INHERITANCE OF ABO BLOOD SYSTEM H gene (FUT1) located on chromosome no 19 in the form of two allele denoted as H and h H is dominant while h is recessive in their pattern of inheritance Hh/HH produce H antigen while rarely hh produce recessive form or amorphic h antigen (Bombay phenotype) It is first time reported in Bombay 13 INHERITANCE OF ABO BLOOD SYSTEM The ABO genes do not code for the production of ABO antigens, but rather produce specific glycosyl transferases ABO produces a specific glycosyl transferases that add sugars to a basic precursor substance on the RBCs 14 INHERITANCE OF ABO BLOOD SYSTEM 15 INHERITANCE OF ABO BLOOD SYSTEM 16 ABO BLOOD GROUP ANTIGENS SYNTHASIS Blood group antigens are actually sugars attached to the red blood cell Individuals inherit a gene which codes for specific sugar(s) to be added to the red cell The type of sugar added determines the blood group 17 ABO BLOOD GROUP ANTIGENS 18 ABO BLOOD GROUP ANTIGENS 19 ABO BLOOD GROUP ANTIBODIES At the birth ABO antibodies are generally absent (3-6 month synthases begin, 5-10 years peak production) ABO antibodies in adults are present in the serum when corresponding ABO antigen is genetically absent Blood group A serum contains anti-B, group B serum contains anti-A, group AB serum contains no antibodies group O serum contains anti-A, anti-B Bombay blood group serum contain anti H, anti A &B 20 ABO BLOOD GROUP ANTIBODIES ABO antibodies are mostly IgM type but IgG and IgA is also present Antibody characteristics Saline reactivity : Yes Thermal amplitude : 4 C, room temperature, 37C Immunoglobulin class : IgM (A&B group), IgG (O group) Complement binding : Yes Placental transfer : Only IgG in group O females Clinically significant : Yes Transfusions reactions : Yes Hemolytic disease of the new born : Yes 21 ABO BLOOD GROUP ANTIBODIES Immediate hemolytic transfusion reaction is seen if an incompatible whole blood or red cell products are transfused to a patient. Complement mediated intravascular hemolysis also takes place ABO antibodies have the ability to react at room temperature (20-24C) or below (4C). They efficiently activate complement at 37C. Thus ABO system is unique among other cold blood groups because of its reactivity at both extremes of temperature. 22 ABO BLOOD GROUP TESTING ABO blood group testing Automated blood Manuel blood group typing group typing with Tile method Tube method Gel card automated analyzer (emergency blood (Forward and method grouping) reverse grouping) Blood group Genotyping/ molecular diagnosis 23 ABO BLOOD GROUP ROUTINE TESTING Commonly tile method, tube method and gel card method is used in most hospitals Tile / slide method is used only in emergency blood grouping not recommended in compatibility testing and blood transfusion Only tube and gel card method is recommended with cell typing (Forward grouping) and serum grouping (Reverse grouping) 24 ABO BLOOD GROUP ROUTINE TESTING Forward grouping by tube method is required known antibodies (antisera) that are specific at detecting a particular ABO antigen on RBCs Reverse grouping by tube method is required known antigens (known red cells) that are specific at detecting a particular ABO antibodies in serum 25 ABO BLOOD GROUP ROUTINE TESTING Antisera manufactured from human sera Antisera used: Antisera Color Source Anti-A Blue Group B donor Anti-B Yellow Group A donor Anti-A,B Red Group O donor 26 ABO BLOOD GROUP ROUTINE TESTING 27 ABO BLOOD GROUP ROUTINE TESTING 28 ABO BLOOD GROUP ROUTINE TESTING Forward Grouping 29 ABO BLOOD GROUP ROUTINE TESTING Reverse Grouping 30 ABO BLOOD GROUP ROUTINE TESTING Patient Red Cells Tested With Patient Anti-A Anti-B 1 0 0 2 4+ 0 3 0 4+ 4 4+ 4+ Interpretation 31 ABO BLOOD GROUP ROUTINE TESTING Patient Red Cells Tested With Patient Anti-A Anti-B Interpretation 1 0 0 O 2 4+ 0 A 3 0 4+ B 4 4+ 4+ AB 32 Thank You Any Question.? 33 Rh Blood Group System Awal Mir Khattak M.Phil MLS, PhD Scholar Rh Blood Group System Introduction: Rhesus (Rh) blood group system, was so named because rhesus monkeys red cell was injected into rabbit and pigs. The antibodies was produced against rhesus monkey red cells which is mostly reacted against human red cell (Rh positive blood group) and some not (Rh negative) Rh antigens are basically trans membrane proteins and most complex blood group system with more than 50 different Rh antigens 3 Rh Blood Group System Historical Background The Rh blood group system was discovered in New York in 1939 with an antibody in the serum of a woman who had given birth to a stillborn baby and then developed a hemolytic reaction as the result of transfusion with blood from her husband. Levine and Stetson found that the antibody agglutinated the red cells of her husband and those of 80% of ABO compatible blood donors 4 Rh Blood Group System Historical Background Unfortunately, Levine and Stetson did not name the antibody In 1940, Landsteiner and Wiener made antibodies by injecting rhesus monkey red cells into rabbits These antibodies not only agglutinated rhesus monkey red cells, but also red cells of white New Yorkers Later this antibodies were labelled as anti-D of the Rh blood group system 5 Rh Blood Group System Molecular genetics of Rh blood group system RHD and RHCE are two closely linked genes located on chromosome no 1 that encode Rh blood group antigens Both genes have 10 exons and share about 94% sequence identity throughout all introns and exons Another gene, SMP1, encoding a small membrane protein, is located between the Rh genes 6 Rh Blood Group System Molecular genetics of Rh blood group system RHD and RHCE encode proteins of 417 amino acids RHD, which encodes the D antigen, and RHCE, which encodes the Cc and Ee antigens The RhD and RhCcEe proteins differ by between 31 and 35 amino acids, according to RHCE allele Rh proteins cross the membrane 12 times, providing six extracellular loops, the potential sites for expression of Rh antigens 7 Rh Blood Group System 8 Rh Blood Group System Rh antigens: Rh antigens are proteins integral to the RBC membrane, passing through the RBC membrane 12 times The antigens are found only on RBCs, and are not soluble or expressed on other cells The Rh antigens are well developed at birth, and as such can cause hemolytic disease of the fetus and newborn 9 Rh Blood Group System Rh antigens: Rh antigens are highly immunogenic, Rh mediated hemolytic transfusion reactions and HDN occurs The five common Rh antigens are D, C, E, c, and e, and their specific corresponding antibodies Rh antigen immunogenicity: D > c > E > C > e The D antigen does not have an allele, but C and c are alleles and E is allelic to e 10 Rh Blood Group System Rh antigens: Rh antigens are proteins integral to the RBC membrane, passing through the RBC wall 12 times The antigens are found only on RBCs, and are not soluble or expressed on other cells The Rh antigens are well developed at birth, and as such can cause hemolytic disease of the fetus and newborn Rh antigens are highly immunogenic, Rh mediated hemolytic transfusion reactions occurs 11 Rh Blood Group System Rh Positive and Rh Negative RHD genes is inherited as co-dominant, when one or two RHD genes inherited that encode Rh-D antigen and typed as a Rh positive Rh-negative phenotypes are so called because the RBCs lack detectable D antigen due to complete deletion of the RHD gene or mutation 12 Rh Blood Group System D antigen polymorphism Numerous variants of D exist, mostly caused by mutations within the RHD gene. D variants have been ranked into two main classes i-e Weak D (formally denoted as Du) and Partial D Weak D in which the whole D antigen is expressed, but expressed weakly in quantity due to genetic inheritance 13 or C trans position effect (Dce/dCe) Rh Blood Group System D antigen polymorphism All D epitopes are present, individuals with weak D cannot make anti-D when immunized by a normal, complete D antigen Weak D is usually associated with amino acid substitutions in the membrane-spanning and are not exposed to the outside of the membrane 14 Rh Blood Group System D antigen polymorphism Partial D, in which part of the D antigen is missing while some D epitopes are expressed and these may be expressed normally or weakly Partial D individuals has missing D epitopes can make an antibody (behaved as antiD) to those epitopes they lack following when immunized by a normal, complete D antigen It is usually associated with amino acid changes in the exposed extracellular loops of the RhD protein 15 Rh Blood Group System Anti D antibodies: Anti-D is clinically the most important red cell antibody in transfusion medicine after anti-A and anti–B Antibody Characteristics: Saline reactivity : Thermal amplitude : Immunoglobulin class : Complement binding : Placental transfer : Clinically significant : Transfusion reactions : Hemolytic disease of the newborn : No 37˚ C IgG No Yes Yes Yes Yes 16 Rh Blood Group System Rh blood group testing Automated blood Manuel blood group typing group typing with Tile method Tube method Gel card automated analyzer method Blood group Genotyping/ molecular diagnosis 17 Rh Blood Group System Rh blood grouping Rh blood grouping is performed parallel with ABO blood grouping Commonly tile, tube and gel card methods are used in most of setups Tile / slide method is used only in emergency blood grouping not recommended in compatibility testing and blood transfusion Only tube and gel card methods are recommended with auto control The anti D anti sera recommended color is transparent or colorless 18 Rh Blood Group System Du grouping / weak D testing It is recommended that all Rh negative blood groups on tile & tube method must be screened for weak D Due to weak D antigens presentation on red blood cells will give no visible agglutination on tile or tube method To detect weak D further tested with anti-human gamma globulin test/coombs test (green color antisera) 19 Rh Blood Group System Du grouping / weak D testing If Rh negative on tile / tube method become positive after addition of coombs antisera is labeled as Du or weak D blood group 20 Rh Blood Group System 21 Rh Blood Group System 22 Thank You Any Question.? 23 HEMOLYTIC DISEASE OF THE NEWBORN Awal Mir M.Phil MLS HEMOLYTIC DISEASE OF NEWBORN Introduction: Hemolytic disease of the newborn (HDNB) is a clincopathological entity characterized by hemolysis, jaundice, anemia and hepatosplenomegaly HDNB denotes an immune hemolysis mediated by trans placental transfer of IgG antibodies formed by the maternal immune system against the antigens on the surface of the fetal red cells which accidentally enter the maternal circulation 3 HEMOLYTIC DISEASE OF NEWBORN Introduction: Accelerated red cell destruction stimulate increases production of red cells ,many of which enter the circulation prematurely as nucleated cells hence the term “erythroblastosis fetalis”. Also called Hydrops fetalis as Severely affected fetuses may develop generalized edema, called “Hydrops fetalis” 4 HEMOLYTIC DISEASE OF NEWBORN Types of HDN: Blood group incompatibility can cause HDN and types depends on which type of blood group incompatible 1. ABO Hemolytic disease of new born (rare) 2. Rh Blood group hemolytic disease of new born (most common) 3. Minor blood group can also causes HDN such as Kell, Kidd and Duffy blood group system 5 HEMOLYTIC DISEASE OF NEWBORN Pathophysiology: In the placenta, maternal and fetal circulations are separated from each other by a semipermeable membrane Under physiological conditions there is virtually no trans placental transfer of red cells between these two circulations At the time of delivery when vessels are ruptured, a small amount of fetal blood (usually no more than 0.1 to 0.2 ml) enters the maternal circulation 6 HEMOLYTIC DISEASE OF NEWBORN Pathophysiology: Similar transfer may take place at the time of abortion, amniocentesis and other transabdominal manipulation This is of no consequence if there is no feto-maternal incompatibility in any of the group systems between the fetus and the mother At times when mother is Rh negative and the fetus is Rh positive, transplacental transfer of fetal red cells (Rh positive) to the maternal circulation (Rh negative) can initiate an immunological process which may have deleterious effects on subsequent pregnancies 7 HEMOLYTIC DISEASE OF NEWBORN Pathophysiology: The first baby invariably escapes ‘un-hurt’ though he has played his role as an inducer of immune response During the next incompatible pregnancy when fetal cells enter the maternal circulation, a secondary response is initiated with rapid, sustained and energetic production of IgG type immune antibodies 8 HEMOLYTIC DISEASE OF NEWBORN Pathophysiology: These sensitized red cells are destroyed by the RES of the fetus and a chain of events is initiated Lead to hemolysis, jaundice hepatosplenomegaly Severity is depends on antigenic exposure, Host factors and antibodies specificity 9 HEMOLYTIC DISEASE OF NEWBORN Pathophysiology: In a group O mother with naturally occurring anti-A and anti-B of the IgG subclass which can cross the placenta. HDN due to ABO incompatibility occurs when a group O mother with IgG anti-A or IgG anti-B is carrying a fetus of blood group A or blood group B respectively. The most common presentation of ABO HDN is jaundice (un-conjugated hyperbilirubinaemia). 10 HDN Pathophysiology 11 HEMOLYTIC DISEASE OF NEWBORN 12 HEMOLYTIC DISEASE OF NEWBORN Clinical Features: Clinical spectrum of HDNB includes Anemia to hydrops fetalis, Hyperbilirubinemi, Hepatosplenomegaly Postnatal problems include: Asphyxia (Unconsciousness) Pallor (due to anemia) Edema (hydrops, due to low serum albumin) Respiratory distress Coagulopathies (↓ platelets & clotting factors) Jaundice Kernicterus (bilirubin encephalopathy) 13 HEMOLYTIC DISEASE OF NEWBORN 14 HEMOLYTIC DISEASE OF NEWBORN Lab Diagnosis : Cord blood parameters: Hemoglobin <16 g/dl High reticulocyte count Baby Rh D positive Direct Coomb’s test positive Indirect Coombs test may also be positive (depending upon the amount of antibody transferred from the mother to the baby). Unconjugated hyperbilirubinemia Normoblastemia Polychromasia Spherocytosis 15 HEMOLYTIC DISEASE OF NEWBORN Normoblastemia on peripheral blood : 16 HEMOLYTIC DISEASE OF NEWBORN Lab Diagnosis : Mother parameters: Rh blood group D negative Circulating anti-D antibodies in the serum 17 HEMOLYTIC DISEASE OF NEWBORN Prevention: Prevention of active immunization Administration of corresponding RBC antibody (e.g anti-D) Use of high-titered Rh-Ig (Rhogam) Calculation of the dose Kleihauer test to evaluate volume of feto-maternal blood and anti D dose calculation 18 HEMOLYTIC DISEASE OF NEWBORN Kleihauer test : It is based on acid elution technique Fetal and maternal RBC have different response to KOH solution Maternal cells (adult Hb ) get eluded leaving behind only cell membrane and hence appear as swollen round large “Ghost Cells “ Normal fetal cells whose Hb remain unaltered hence look as red refractile round cells due to HbF which resist to acid solution (KoH) 19 Thank You Any Question.? 20 TRANSFUSION HISTORY BLOOD PRODUCTS Awal Mir M.Phil MLS, PhD Scholar TRANSFUSION HISTORY The first successful animal to animal transfusion, was performed by Richard Lower at Oxford in February 1665 Direct transfusion from the carotid artery of one dog to the jugular vein of another by insertion of quills into the blood vessels The first animal to human transfusion was performed by Denis in 1667 3 TRANSFUSION HISTORY He administered the blood of a lamb to a 15-year old boy named Arthur Coga Blundell (1790-1877) was the first to state clearly that only human blood should be used for human transfusion The first well-documented transfusion with human blood took place on September 26, 1818 Thus beginning of modern transfusion ere 4 TRANSFUSION HISTORY 5 TRANSFUSION HISTORY In 1900, Karl Landsteiner observed that the sera of some persons agglutinated the red blood cells of others He identify ABO blood groups A, B, and O for the first time In 1913, Ottenberg suggested preliminary blood testing, uses of anticoagulant will reduces transfusion “accidents The M, N, and P systems were described in the period 6 between 1927 and 1947 TRANSFUSION HISTORY The Rh system was discovered in connection with an unusual transfusion reaction. In 1939, Levine and Stetson A blood transfusion service, organized by the Republican Army during the Spanish Civil War (1936-1939) Component and derivative therapy began during World War II 7 TRANSFUSION HISTORY Blood components not only saved lives but also transmitted diseases (HBV, HCV, HIV) So safe blood transfusion concept was established Safe blood which does not harm the patient during or after transfusion of blood 8 BLOOD TRANSFUSION Transfusion medicines or transfusion therapy” is a broad term that comprises all aspects of the transfusion of patients Blood and blood products are considered drugs because of their use in treating diseases As with drugs, adverse effects may occurs It is most dangerous medicines, never transfuse unless it is life saving 9 BLOOD TRANSFUSION Steps in safe blood transfusion Right doctor request (complete bio-data, transfusion history & transfusion reaction, blood component & quantity) Right blood donor (Volunteer blood donor & autologous) Right laboratory tests (Blood group, cross match, antibodies screening, TTIs screening) Right blood component Right Report Right Patient 10 BLOOD TRANSFUSION Steps & requirements in blood administration Recipient/patient consent Patient education and history Medical Order/Consultant advised Laboratory test reports Availability of emergency medicines & equipment's 11 BLOOD TRANSFUSION Recipient/patient consent Recipient consent for the transfusion must be obtained from patients The recipient consent document should contain indications, risks, possible side effects The patient has the right to accept or reject a transfusion If the patient is unable to give consent, a legally authorized representative or surrogate may provide consent 12 BLOOD TRANSFUSION 13 BLOOD TRANSFUSION Patient education and history The patient should have the opportunity to ask questions It is important to take a history from the patient before the component is ordered so that it can be determined if the patient had reactions to components in the past Patient must be educated regarding transfusion reactions occurrence and initial inset appearance 14 BLOOD TRANSFUSION 15 BLOOD TRANSFUSION Medical Order/Consultant advised There must be an order from a licensed care provider and will clearly mentioned required blood component types (RCC, Platelets or FFPs ) and number of unites In order sheet patient, name, age, date of birth and medical history Any special processing required of the component (e.g. washing, irradiation, or filtration or apheresis) The date and time of transfusion 16 BLOOD TRANSFUSION Laboratory reports After receiving an order from a licensed provider, the transfusion service initiates a series of steps to ensure the provision of a compatible component This includes ABO and Rh typing of the patient Cross match report: Donor blood must be matched with recipient Antibody screening report Transfusion transmitted infections screening report (HCV, HBV, HIV, Syphilis and malarial parasite) 17 BLOOD TRANSFUSION 18 BLOOD TRANSFUSION 19 WHOLE BLOOD & BLOOD PRODUCTS Whole Blood Red blood cell concentrate (RCC) Platelets and Platelets apheresis Fresh frozen plasma Cryoprecipitate and cryo-supernatant Apheresis granulocytes 20 WHOLE BLOOD & BLOOD PRODUCTS Whole Blood When no blood components are removed is called whole blood 16 to 24 hours old blood does not contain functional platelets and important coagulation factors like factor V and VIII and must be blood group specific Storage Temperature: 2 to 6 C Shelf Life: 35 days 21 WHOLE BLOOD & BLOOD PRODUCTS Whole Blood One point raises: 1-1.5 mg/dl or 3-5% HCT in adult Albumin content: 10-12 grams Transfusion Time: Should end 3 to 6 hours after issuance Indications for whole blood transfusion: Symptomatic anemia with large-volume deficit (>30-40% blood loss) and exchange blood transfusion 22 WHOLE BLOOD & BLOOD PRODUCTS Whole Blood disadvantages Delayed increase in hemoglobin level Volume overload Electrolyte over load Anticoagulant toxicity Transfusion reactions to plasma proteins Cannot save three lives 23 WHOLE BLOOD & BLOOD PRODUCTS Red Cells Concentrate or packed red blood cells When 80% plasma is removed from whole blood the remaining component is called packed red cells/RCC 70% patients needs RCC Storage Temperature: 2 to 6 C Shelf Life: 35 days Indication: To treat all types of anemia Hb raised: 1-1.5 gm./dl in adults 24 WHOLE BLOOD & BLOOD PRODUCTS Advantages of RCC Hemoglobin is raised more quickly (2 hours) because adjustment in blood volume is less as compared to whole blood Circulatory overload is minimize Volume of anticoagulant & electrolytes is reduced Transfusion reactions to plasma proteins is reduced One blood donation can save three lives 25 WHOLE BLOOD & BLOOD PRODUCTS Leuko-depleted RCC Average unit of RCC contain 2 x 10^9/L WBCs It is achieved by washing method, filtration and irradiation WBCs can causes Allo-immunization Graft versus host disease TRALI Febrile non hemolytic transfusion reaction 26 WHOLE BLOOD & BLOOD PRODUCTS Leuko-depleted RCC indication Severe allergic reactions Beta thalassemia major patients Anaphylactic reactions due to anti IgA Allo-immunization to WBCs which make causes graft verses host disease 27 WHOLE BLOOD & BLOOD PRODUCTS Fresh Frozen Plasma (FFPs) Liquid portion from whole blood is separated and frozen immediately known as FFPs Volume: 150-225 ml Storage Temperature: -40 or -80 C Shelf life: up to 1 years Adult dose: 4-6 unites (12-15ml/kg for paeds) Duration of transfusion: with in two hours Factor VIII level: 80 IU 28 WHOLE BLOOD & BLOOD PRODUCTS Fresh Frozen Plasma (FFPs) Indications of FFPs Snike bite DIC Vitamin K deficiency Hemophilia Contra indication: replacement Volume expansion / protein 29 WHOLE BLOOD & BLOOD PRODUCTS Cryo-Precipitate and cryo-supernatant When FFPs is partially thawed the supernatant liquid is called cryo-supernatant while crystal sediments is called cryoprecipitate Cryoprecipitate Plasma volume: 10-20 mL Shelf Life: 8-12 hours Storage: -80 C for 12 months Factor VIII level: 80 IU, Fibrinogen level: 150-250 mg 30 WHOLE BLOOD & BLOOD PRODUCTS Cryo-Precipitate and cryo-supernatant Cryoprecipitate: Indications Hemophilia A Von Willibrand disease Acquired FVIII deficiency Fibrinogen deficiency Cryo-supernatant is indicated for Hemophilia B, and Albumen replacement 31 WHOLE BLOOD & BLOOD PRODUCTS Platelets concentrate Platelets concentrate is a important blood product and each random bag should contain 0.55 x 10^11 Platelets concentrate volume: 50-60 mL Shelf Life: 3-5 days Storages temperature: 22 C on agitator Adult dose: 4-6 unites Count raised: 5000-10000 in adults Group Specific 32 WHOLE BLOOD & BLOOD PRODUCTS Guideline for therapeutic Platelets Active bleeding with platelets count: <50 x 10^9/L Active bleeding with platelets function defect Micro-vascular bleeding and platelets count <100 x 10^9/L in CABG surgery Bleeding after massive transfusion 33 WHOLE BLOOD & BLOOD PRODUCTS Therapeutic Platelets contraindication Thrombotic thrombocytopenic purpura Post transfusion purpura Idiopathic thrombocytopenic purpura Heparin induced thrombocytopenic purpura 34 Thank You Any Question.? 35 TRANSFUSION REACTIONS TRANSFUSION TRANSMITTED INFECTIONS Awal Mir M.Phil MLS, PhD Scholar TRANSFUSION REACTIONS Any adverse events associated with the transfusion of whole blood or one of the its component These reactions in severity from minor to life threatening Reactions can occurs during the transfusion is termed acute transfusion reactions If reactions do happened days to week later is termed delayed transfusion reactions 3 TRANSFUSION REACTIONS Transfusion reaction may be immunologic and non immunologic Transfusion reaction also depends on blood product types Red cells causes hemolytic transfusion reactions, malaria and babesia (cross match required) Platelets causes HLA allo-immunization, post 4 transfusion purpura (cross match not required) TRANSFUSION REACTIONS WBCs causes fever, HLA allo-immunization, graft verses host disease, transfusion related acute lung injury, transmitting cytomegaly virus (cross match not required) Plasma causes allergic reactions, anaphylactic, volume overload, anticoagulant toxicity, hypocalcaemia, electrolytes imbalance (cross match not required) 5 TRANSFUSION REACTIONS 6 ABO BLOOD GROUP TESTING Transfusion Reactions Types Delayed transfusion reactions Immediate or acute transfusion reactions • AHTRs Non Immune mediated Immune mediated • Transfusion related sepsis • DHTRs • FNHTR • Non immune hemolysis • TAGVHD • Urticarial • Air embolism • Post transfusion • Anaphylactic • Transfusion associated Immune mediated • TRALI circulatory over load Non-Immune • Iron overload purpura • Allo-immunization of RBC, Platelets, 7 WBC TRANSFUSION REACTIONS 8 TRANSFUSION REACTIONS 9 TRANSFUSION REACTIONS 10 TRANSFUSION REACTIONS Transfusion reactions may also common reactions, less common reactions and unusual reactions Common reactions: Febrile, allergic, allo-immunization Less common reactions: Circulatory overload, delyed transfusion reactions, depletion of coagulation factors and platelets Unusual reactions: anaphylactic, TRALI, transfusion purpura Immediate transfusion bacterial contamination, reactions, GVHD, 11 TRANSFUSION REACTIONS 12 TRANSFUSION REACTIONS 13 TRANSFUSION TRANSMITTED INFECTIONS Blood is a lifesaving resource, However bacterial, viral, parasitic, and prion pathogens constantly transmitted via blood transfusion. If it is not detected in the testing process, can cause harm and even death So donor must be properly screened for transfusion transmitted pathogens with high sensitive laboratory techniques. 14 TRANSFUSION TRANSMITTED INFECTIONS Common transfusion transmitted pathogens Hepatitis B Virus (HBV) Hepatitis C Virus (HCV) Human Immunodeficiency Virus (HIV) T. Pallidum (Bacteria) Malarial Parasite (Blood parasite) 15 TRANSFUSION TRANSMITTED INFECTIONS Rare transfusion transmitted pathogens Human T-cell lymphotropic virus types I and II (anti-HTLVI/II) Epstein Barr Virus (EBV) Cytomegaly Virus (CMV) Parvovirus B19 (B19) West Nile virus Trypanosoma cruzi Babesia microti 16 TRANSFUSION TRANSMITTED INFECTIONS HBV and HCV causes transfusion associated hepatitis HIV causes acquired immunodeficiency syndrome T. Pallidum (Bacteria) causes Syphilis Malarial Parasite (Blood parasite) causes malaria fever EBV causes infectious mononucleosis (kissing disease CMV causes mononucleosis and abortion in pregnant women 17 TRANSFUSION TRANSMITTED INFECTIONS HBV and HCV causes transfusion associated hepatitis HIV causes acquired immunodeficiency syndrome T. Pallidum (Bacteria) causes Syphilis Malarial Parasite (Blood parasite) causes malaria fever EBV causes infectious mononucleosis (kissing disease CMV causes mononucleosis and abortion in pregnant women 18 TRANSFUSION TRANSMITTED INFECTIONS Laboratory diagnostic Techniques uses for TTIs Immuno-chromate-graphic technique (ICT) Enzyme linked immuno-sorbent assay (ELISA) Chemiluminescence assay (CIMA) Polymerase Chain Reactions (PCR) Western Blotting for HIV conformation Peripheral blood film examination for blood parasites 19 TRANSFUSION TRANSMITTED INFECTIONS 20 Thank You Any Question.? 21