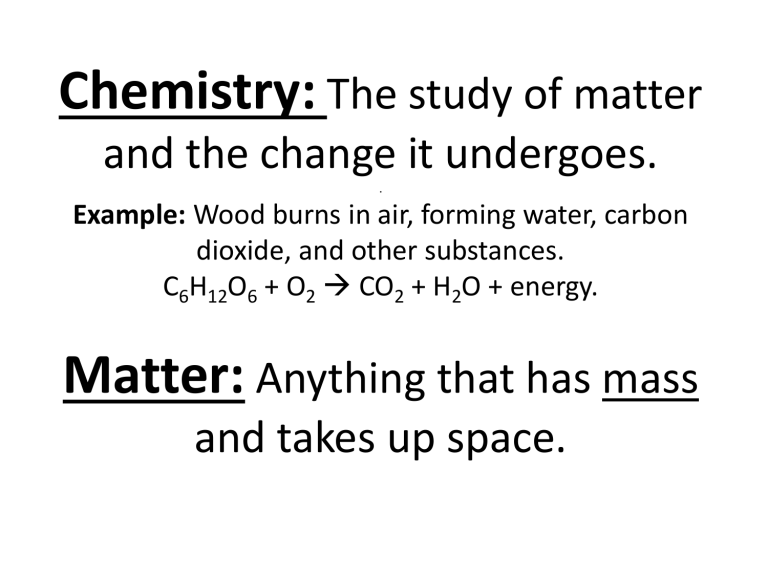
Chemistry: The study of matter and the change it undergoes. . Example: Wood burns in air, forming water, carbon dioxide, and other substances. C6H12O6 + O2 → CO2 + H2O + energy. Matter: Anything that has mass and takes up space. Macroscopic and Microscopic understanding of the universe. We can observe and describe properties that are both macroscopic and microscopic for water. Matter is composed of atoms. Georges Seurat – Sunday Afternoon Atom: Tiny particles composed of protons, neutrons, and electrons. Compounds: Bonding atoms together in specific ways. Elements: Pure substance containing one type of atom.



