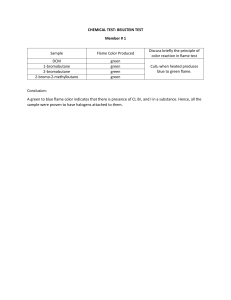
AAS AND ICP: 1. How is the analyte concentration determined in atomic spectroscopy? The essential idea in atomic spectroscopy is to measure the concentration of a metal in a sample, by burning the sample (atomizing the sample) and then perform spectroscopy on the sample. The spectroscopy part is performed with UV/VIS light because atoms do not have any rotational motion or vibrational motion, in other words, atoms and their electronic energy levels are the subject of interest. Before being able to understand how atomic absorption spectroscopy works, we must be able to understand a little bit about spectroscopy in general. Spectroscopy concerns the interaction between radiation and matter. Electrons in an atom can absorb discrete amounts of energy provided by light and move from one orbital or another when “excited”. The energy that an electron absorbs must be equal to the amount of energy needed to move up to a certain orbital, giving each element has its own fingerprint if you like. In other words, orbits of different elements have different energy levels, which causes the amount of energy an electron can absorb to be different between elements, making them distinguishable. When these excited electrons return to their ground state, they will emit the same amount of energy they received before excitation. This energy is released in the form of a photon. This electromagnetic radiation is also specific to an element, which now brings us to atomic spectroscopy. Atomic absorption spectroscopy (AAS) is a quantitative analysis of metal ions. The amount of energy absorbed determines the quantity of metal ions, in other words, the more metal ions, the greater the absorption. The relationship between the amount of metal ion or metal atom present in a sample and the extent in which the electromagnetic radiation is absorbed is described by the Beer-Lambert law. It states that the absorption of electromagnetic radiation is directly proportional to the concentration of metal ions or atoms present in the given sample. The higher the concentration of metal ions or atoms, the more absorbance will occur. Therefore, by measuring the amount of light absorbed, we can determine the concentration of a particular metal in the sample. We start out by making a calibration curve with known concentrations, Then, with the help of regression, we can make a formula for the absorbance. 𝐴=𝑎∗𝐶+𝑏 A = absorbance. C = concentration Then when we have analyzed our sample, we can insert the absorbance in the standard curve and thereby calculate the unknown concentration. But before we analyze the concentration of a particular metal using AAS, we need to know the specific wavelength that metal can absorb. The energy of electromagnetic radiation is directly proportional to the frequency. In other words, the higher the frequency the more energy. Also, the more energy the shorter the wavelength. The hollow cathode lamp produces the EMR (Electromagnetic radiation) that is required for exciting metal atoms. The cathode of this lamp is always made from the same metal element as the subject of interest, this is a very important requirement. The metal atoms of the cathode are made to be excited, which means the electrons energy absorb energy an excited state orbital. When these electrons return to the ground state, the EMR released is specific to the metal. The nebulizer sprays the liquid sample containing the metal of interest into the flame, which atomizes and converts the metal species into metal atoms in the ground state. The EMR from the hollow cathode lamp is passed through the hottest part of the flame and is absorbed by the atomized metals of interest. Due to the absorbance of EMR by the metal atoms in the flame, the amount of EMR before the flame, must be greater than the amount of EMR after the flame. We can describe this using the intensity of EMR. So: the original intensity of EMR 𝐼0 , is greater than the new intensity of EMR coming out of the flame 𝐼. Beer-Lambert Law tells us that the extent to which EMR is absorbed, depends on the concentration of a metal in the sample. In other words, when there are more atomized metal atoms in the flame, more EMR will be absorbed, causing less EMR to exit the flame. This means, the final intensity of the EMR, 𝐼, will be smaller than 𝐼0 . In other words: 𝐼0 > 𝐼 All this can be summarized using this mathematical formula that is Beer-Lambert’s law: 𝐼0 𝐴 = 𝜀𝑐𝑏 = log ( ) 𝐼 Here the absorbance is equal to the log function of: 𝐼0 /𝐼 . This logarithmic expression also equals 𝜀𝑐𝑏 . Here epsilon is known at the extinction coefficient which tells us about the absorptive properties of the analyte of interest. Concentration of a sample is expressed as c. And finally, b, is known as the pathlength, here that is the distance light must travel through the flame. The monochromator receives the EMR with the wavelength emitted by the hollow cathode lamp, eliminates the background EMR (such as visible light) and sends the correct wavelength only to the detector. Finally, the detector measures the intensity of the received electromagnetic radiation to calculate the absorbance. A calibration curve is set up in advance, with many standard solutions of variating known concentrations and is plotted together on an absorbance vs concentration (ppm) graph. Using the line of best fit, concentration can be calculated from here. 2) What is the difference between atom absorption, fluorescence and emission? 1. Atom emission: the atoms in a flame are excited by the high temperature and emit light 2. Atom absorption: the atoms in a flame absorbs a part of the light from a lamp and the rest reaches a detector 3. Atomic fluorescence: the atoms in the flame are excited by a laser, the excited atoms will then return to ground state under light emissions. 3) How are the analytes from aqueous solutions atomized? Describe the principles and the differences between the flame, graphite oven and plasma methods? An aqueous solution is atomized, by first reducing the solvent followed by volatilizing the analyte and then divide the sample into free atoms. Excess oxidant is blown over the sample capillary entrance, which breaks up the liquid sample into small droplets. These droplets are then further nebulized by either a glass bead (in flame spectroscopy) or with a nebulizer (in ICP). The resulting aerosols are further mixed, and larger droplets are separated until around 5% of the original sample reaches the flame. Analytes in aqueous solutions are atomized differently by the three methods. 1. The flame method is characterized by the nebulization of the liquid solution mixed with the oxidant and the fuel. When the mix reaches the flame, it contains only about 5% of the initial sample. The droplets entering the flame evaporate and the remaining solid evaporates and decomposes into atoms. 2. The electrically heated graphite furnace requires less sample and is more sensitive than a flame. This is because the residence time of analytes in the optical path is several seconds, whereas for the flame is less than 1 second. Compared with flames, furnaces require more operator skill since the sample needs to be injected precisely on the floor of the furnace, not too high or too late. The samples are dried, decomposed with heat, a phenomenon called pyrolysis, and finally atomized. 3. The inductively coupled plasma is twice as hot as a combustion flame. The high temperature combined with stability and relatively inert Argon environment eliminate most of the interference encountered with flames. The samples are pumped slowly into the nebulizer. The fine mist exiting the top of the chamber reaches the plasma torch as an aerosol of dry particles. Since the plasma energy doesn’t need to evaporate the sample, more energy is available to atomize it. The sensitivity is 3 to 10 times higher when the emission is observed along the length of the plasma. 4) Review the instrumentation of the AAS flame technique including: a) atomic line widths and b) hollow cathode lamp. In atomic absorption spectroscopy flame technique, the liquid sample is aspirated into a flame and atomized. In the hollow-cathode lamp, the cathode is bombarded with Ne+ or Ar+ ions, which excited emits the same frequencies absorbed by the analyte in the flame. On the other side of the flame a detector measures the amount of light that passed through the flame. The linewidth of the source must be narrower than the linewidth of the atomic vapor for Beer’s law to be obeyed. So, The cathode is made of the element with the desired emission lines to be observed. Hallow-cathode lamp is used in atomic absorption spectroscopy to produce narrower lines of correct frequency compared to monochromators. This is because the atoms in the lamp are cooler than atoms in the flame, so lamp emission is sufficiently narrower than the absorption line width of atoms in the flame. 5) Describe the significance of the temperature for atomic spectroscopy, including the effect on a) the excited state population b) the absorption and emission signal Temperature determines how much a sample will break down into atoms and the extend to which a given atom is found in its different states (ground, excited or ionized). An increase of temperature increases the excited state population, following the Boltzmann distribution equation: T is in Kelvin and the Boltzmann constant is 1.381 ∗ 10( − 23) J/K The Boltzman distribution describes the relative population of the different states at thermal equilibrium. The result shows how big a part of the atoms is in the exited state. Emission intensity is proportional to the population of the excited state. Absorption arises from ground-state atoms, but emission arises from excited-state atoms. 6) The graphite oven technique is not included in this exercise, but how does a typical heating profile for the atomization using a graphite oven look like? a) Why can it be necessary to use a matrix modifier? Everything in a sample other than analyte is called matrix. Ideally the matrix decomposes and vaporizes during the charring step. A matrix modifier is a substance added to the sample to reduce the loss of analyte during charring by making the matrix more volatile, or making analyte less volatile b) Why are there generally obtained lower detection limits for the graphite oven method? The detection limit for furnaces is typically two orders of magnitude lower (they differ by a factor of about 100) than that observed with a flame, because the sample is confined in the small volume of the furnace, for a relatively long time, which gives the high sensitivity. Generally lower detection limits are obtained for the graphite oven method since the sample is confined in the optical path for several seconds. 7) Explain the instrumentation for the ICP and explain how multiple detection of different elements works? An inductively coupled plasma emission spectrometer does not require any lamps and can measure up to 70 elements simultaneously. 8) Why is it necessary to perform background correction and how is this done? It is important for atomic spectroscopy to provide background correction to distinguish analyte signal from the background absorption, emission, and optical scattering caused by the sample matrix, flame, plasma, or red-hot graphite furnace. Therefore, background correction excludes background absorbance and thus formation of any significant errors. In general, background correction is done by measuring the signals obtained from the analyte versus the ones obtained from the background and subtracting the values to determine the corrected signal. This procedure can be carried out by different methods such as, subtraction of adjacent pixels of CID display, beam chopping, deuterium lamp correction and Zeeman effect application. 9) What types of interferences are found in atomic spectroscopy and for which method are they a problem? Spectral interference, Chemical interference, and Ionization interference In absorption, we see a low spectral inference, but a high chemical interference. And in Atomic emission we see a high spectral interference, but a lower chemical interference. However, ICP also suffers from ionization interference. 10) Compare the detection limit for the different methods of atomic spectroscopy. As seen in the picture ICP/mass spectrometry has a very low detection limit, which make it very precise. ICP/atomic emission (pneumatic nebulizer) has a high detection limit, which can be problematic. 11) Discuss the pros and cons for the different methods of atomic spectroscopy. ICP: • Use of plasma eliminates many common interferences, for example, flames emit light that must be subtracted from the total signal to obtain the analyte signal. The inductively coupled plasma is twice as hot as a combustion flame. The high temperature, stability, and relatively inert Ar environment eliminate much of the interference encountered with flames. Simultaneous multielement analysis is routine for inductively coupled plasma–atomic emission spectroscopy, which has replaced flame atomic absorption. However, the plasma instrument costs more to purchase and operate than a flame instrument. But formation of oxides / hydroxides is negligible. The plasma is free of background radiation. • Plasma energy is not needed to evaporate solvent, so more energy is available for atomization. Furnace: • An electrically heated graphite furnace is more sensitive than a flame and requires less sample. In flame spectroscopy, the residence time of analyte in the optical path is <1 second as it rises through the flame. A graphite furnace confines the atomized sample in the optical path for several seconds, thereby affording higher sensitivity. • Compared with flames, furnaces require more operator skill to find proper conditions for each type of sample. • Interference from previous runs, called a memory effect may occur. However, it is reduced in a transversely heated furnace and to further reduce memory effects, ordinary graphite is coated with a dense layer of pyrolytic carbon formed by thermal decomposition of an organic vapor. The coating seals the relatively porous graphite to reduce absorption of foreign atoms. • A sample can be preconcentrated by injecting and evaporating multiple aliquots in the graphite furnace prior to analysis. This results in lower detection limit, which is favorable when working with substances that are hazardous in small concentrations. • Liquid samples are ordinarily used in furnaces. In direct solid sampling, a solid is analyzed without sample preparation. However, more sample is analyzed when solid is injected than when liquid is injected, and therefore detection limits can be 100 times lower than those obtained for liquid injection. Flame: • Flames emit light that must be subtracted from the total signal to obtain the analyte signal. • If the flame is relatively rich in fuel (a “rich” flame), excess carbon tends to reduce metal oxides and hydroxides and thereby increases sensitivity. A “lean” flame, with excess oxidant, is hotter. Different elements require either rich or lean flames for best analysis. • Many elements form oxides and hydroxides in the outer cone. • Molecules do not have the same spectra as atoms, so the atomic signal is lowered. Molecules also emit broad radiation that must be subtracted from the sharp atomic signals. • The height in the flame at which maximum atomic absorption or emission is observed depends on the element being measured and the flow rates of sample, fuel, and oxidizer.